Reporting guidelines for survey research: an analysis of published guidance and reporting practices
- PMID: 21829330
- PMCID: PMC3149080
- DOI: 10.1371/journal.pmed.1001069
Reporting guidelines for survey research: an analysis of published guidance and reporting practices
Abstract
Background: Research needs to be reported transparently so readers can critically assess the strengths and weaknesses of the design, conduct, and analysis of studies. Reporting guidelines have been developed to inform reporting for a variety of study designs. The objective of this study was to identify whether there is a need to develop a reporting guideline for survey research.
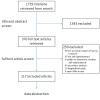
Methods and findings: We conducted a three-part project: (1) a systematic review of the literature (including "Instructions to Authors" from the top five journals of 33 medical specialties and top 15 general and internal medicine journals) to identify guidance for reporting survey research; (2) a systematic review of evidence on the quality of reporting of surveys; and (3) a review of reporting of key quality criteria for survey research in 117 recently published reports of self-administered surveys. Fewer than 7% of medical journals (n = 165) provided guidance to authors on survey research despite a majority having published survey-based studies in recent years. We identified four published checklists for conducting or reporting survey research, none of which were validated. We identified eight previous reviews of survey reporting quality, which focused on issues of non-response and accessibility of questionnaires. Our own review of 117 published survey studies revealed that many items were poorly reported: few studies provided the survey or core questions (35%), reported the validity or reliability of the instrument (19%), defined the response rate (25%), discussed the representativeness of the sample (11%), or identified how missing data were handled (11%).
Conclusions: There is limited guidance and no consensus regarding the optimal reporting of survey research. The majority of key reporting criteria are poorly reported in peer-reviewed survey research articles. Our findings highlight the need for clear and consistent reporting guidelines specific to survey research.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- Groves RM, Fowler FJ, Couper MP, Lepkowski JM, Singer E, et al. Hoboken (New Jersey): John Wiley & Sons, Inc; 2004. Survey Methodology.
-
- Aday LA, Cornelius LJ. Hoboken (New Jersey): John Wiley & Sons, Inc; 2006. Designing and Conducting Health Surveys.
-
- McColl E, Jacoby A, Thomas L, Soutter J, Bamford C, et al. Design and use of questionnaires: a review of best practice applicable to surveys of health service staff and patients. Health Technol Assess. 2001;5:1–256. - PubMed
-
- Simera I, Moher D, Hoey J, Schulz KF, Altman DG. The EQUATOR Network and reporting guidelines: Helping to achieve high standards in reporting health research studies. Maturitas. 2009;63:4–6. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources