Two group A streptococcal peptide pheromones act through opposing Rgg regulators to control biofilm development
- PMID: 21829369
- PMCID: PMC3150281
- DOI: 10.1371/journal.ppat.1002190
Two group A streptococcal peptide pheromones act through opposing Rgg regulators to control biofilm development
Erratum in
- PLoS Pathog. 2011 October 10; 7(10): 10.1371/annotation/a41fff48-2a84-4cb8-b27c-afd14bcd40f0
Abstract
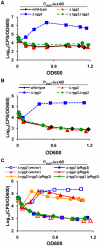
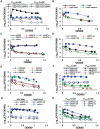
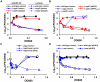
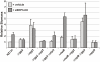
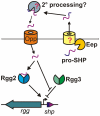
Streptococcus pyogenes (Group A Streptococcus, GAS) is an important human commensal that occasionally causes localized infections and less frequently causes severe invasive disease with high mortality rates. How GAS regulates expression of factors used to colonize the host and avoid immune responses remains poorly understood. Intercellular communication is an important means by which bacteria coordinate gene expression to defend against host assaults and competing bacteria, yet no conserved cell-to-cell signaling system has been elucidated in GAS. Encoded within the GAS genome are four rgg-like genes, two of which (rgg2 and rgg3) have no previously described function. We tested the hypothesis that rgg2 or rgg3 rely on extracellular peptides to control target-gene regulation. We found that Rgg2 and Rgg3 together tightly regulate two linked genes encoding new peptide pheromones. Rgg2 activates transcription of and is required for full induction of the pheromone genes, while Rgg3 plays an antagonistic role and represses pheromone expression. The active pheromone signals, termed SHP2 and SHP3, are short and hydrophobic (DI[I/L]IIVGG), and, though highly similar in sequence, their ability to disrupt Rgg3-DNA complexes were observed to be different, indicating that specificity and differential activation of promoters are characteristics of the Rgg2/3 regulatory circuit. SHP-pheromone signaling requires an intact oligopeptide permease (opp) and a metalloprotease (eep), supporting the model that pro-peptides are secreted, processed to the mature form, and subsequently imported to the cytoplasm to interact directly with the Rgg receptors. At least one consequence of pheromone stimulation of the Rgg2/3 pathway is increased biogenesis of biofilms, which counteracts negative regulation of biofilms by RopB (Rgg1). These data provide the first demonstration that Rgg-dependent quorum sensing functions in GAS and substantiate the role that Rggs play as peptide receptors across the Firmicute phylum.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- Wilson M. New York: Cambridge University Press. xviii, 455 p; 2005. Microbial inhabitants of humans : their ecology and role in health and disease.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

