Pancreatic β-cell death in response to pro-inflammatory cytokines is distinct from genuine apoptosis
- PMID: 21829464
- PMCID: PMC3146470
- DOI: 10.1371/journal.pone.0022485
Pancreatic β-cell death in response to pro-inflammatory cytokines is distinct from genuine apoptosis
Abstract
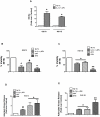
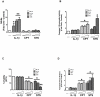
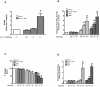
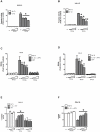
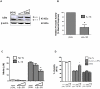
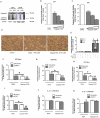
A reduction in functional β-cell mass leads to both major forms of diabetes; pro-inflammatory cytokines, such as interleukin-1beta (IL-1β) and gamma-interferon (γ-IFN), activate signaling pathways that direct pancreatic β-cell death and dysfunction. However, the molecular mechanism of β-cell death in this context is not well understood. In this report, we tested the hypothesis that individual cellular death pathways display characteristic phenotypes that allow them to be distinguished by the precise biochemical and metabolic responses that occur during stimulus-specific initiation. Using 832/13 and INS-1E rat insulinoma cells and isolated rat islets, we provide evidence that apoptosis is unlikely to be the primary pathway underlying β-cell death in response to IL-1β+γ-IFN. This conclusion was reached via the experimental results of several different interdisciplinary strategies, which included: 1) tandem mass spectrometry to delineate the metabolic differences between IL-1β+γ-IFN exposure versus apoptotic induction by camptothecin and 2) pharmacological and molecular interference with either NF-κB activity or apoptosome formation. These approaches provided clear distinctions in cell death pathways initiated by pro-inflammatory cytokines and bona fide inducers of apoptosis. Collectively, the results reported herein demonstrate that pancreatic β-cells undergo apoptosis in response to camptothecin or staurosporine, but not pro-inflammatory cytokines.
Conflict of interest statement
Figures


Similar articles
-
Distinct differences in the responses of the human pancreatic β-cell line EndoC-βH1 and human islets to proinflammatory cytokines.Am J Physiol Regul Integr Comp Physiol. 2015 Sep;309(5):R525-34. doi: 10.1152/ajpregu.00544.2014. Epub 2015 Jun 17. Am J Physiol Regul Integr Comp Physiol. 2015. PMID: 26084699 Free PMC article.
-
Induction of nuclear factor-kappaB and its downstream genes by TNF-alpha and IL-1beta has a pro-apoptotic role in pancreatic beta cells.Diabetologia. 2008 Jul;51(7):1213-25. doi: 10.1007/s00125-008-0999-7. Epub 2008 May 8. Diabetologia. 2008. PMID: 18463842
-
Effect of iNOS and NF-kappaB gene silencing on beta-cell survival and function.J Drug Target. 2007 Jun;15(5):358-69. doi: 10.1080/10611860701349695. J Drug Target. 2007. PMID: 17541845
-
Mechanisms of pancreatic beta-cell death in type 1 and type 2 diabetes: many differences, few similarities.Diabetes. 2005 Dec;54 Suppl 2:S97-107. doi: 10.2337/diabetes.54.suppl_2.s97. Diabetes. 2005. PMID: 16306347 Review.
-
Role of NF-kappaB in beta-cell death.Biochem Soc Trans. 2008 Jun;36(Pt 3):334-9. doi: 10.1042/BST0360334. Biochem Soc Trans. 2008. PMID: 18481952 Review.
Cited by
-
CCL20 is elevated during obesity and differentially regulated by NF-κB subunits in pancreatic β-cells.Biochim Biophys Acta. 2015 Jun;1849(6):637-52. doi: 10.1016/j.bbagrm.2015.03.007. Epub 2015 Apr 13. Biochim Biophys Acta. 2015. PMID: 25882704 Free PMC article.
-
CXC chemokine ligand 12 protects pancreatic β-cells from necrosis through Akt kinase-mediated modulation of poly(ADP-ribose) polymerase-1 activity.PLoS One. 2014 Jul 2;9(7):e101172. doi: 10.1371/journal.pone.0101172. eCollection 2014. PLoS One. 2014. PMID: 24988468 Free PMC article.
-
Regulation of iNOS gene transcription by IL-1β and IFN-γ requires a coactivator exchange mechanism.Mol Endocrinol. 2013 Oct;27(10):1724-42. doi: 10.1210/me.2013-1159. Epub 2013 Sep 6. Mol Endocrinol. 2013. PMID: 24014650 Free PMC article.
-
Oral Corticosterone Administration Reduces Insulitis but Promotes Insulin Resistance and Hyperglycemia in Male Nonobese Diabetic Mice.Am J Pathol. 2017 Mar;187(3):614-626. doi: 10.1016/j.ajpath.2016.11.009. Epub 2017 Jan 4. Am J Pathol. 2017. PMID: 28061324 Free PMC article.
-
β-Cell-selective regulation of gene expression by nitric oxide.Am J Physiol Regul Integr Comp Physiol. 2024 Jun 1;326(6):R552-R566. doi: 10.1152/ajpregu.00240.2023. Epub 2024 Apr 8. Am J Physiol Regul Integr Comp Physiol. 2024. PMID: 38586887 Free PMC article.
References
-
- Mathis D, Vence L, Benoist C. beta-Cell death during progression to diabetes. Nature. 2001;414:792–798. - PubMed
-
- Mandrup-Poulsen T. The role of interleukin-1 in the pathogenesis of IDDM. Diabetologia. 1996;39:1005–1029. - PubMed
-
- Corbett JA, Wang JL, Sweetland MA, Lancaster JR, Jr, McDaniel ML. Interleukin 1 beta induces the formation of nitric oxide by beta-cells purified from rodent islets of Langerhans. Evidence for the beta-cell as a source and site of action of nitric oxide. J Clin Invest. 1992;90:2384–2391. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

