Endothelium-derived Netrin-4 supports pancreatic epithelial cell adhesion and differentiation through integrins α2β1 and α3β1
- PMID: 21829502
- PMCID: PMC3146510
- DOI: 10.1371/journal.pone.0022750
Endothelium-derived Netrin-4 supports pancreatic epithelial cell adhesion and differentiation through integrins α2β1 and α3β1
Abstract
Background: Netrins have been extensively studied in the developing central nervous system as pathfinding guidance cues, and more recently in non-neural tissues where they mediate cell adhesion, migration and differentiation. Netrin-4, a distant relative of Netrins 1-3, has been proposed to affect cell fate determination in developing epithelia, though receptors mediating these functions have yet to be identified.
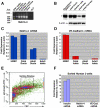
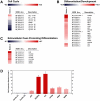
Methodology/principal findings: Using human embryonic pancreatic cells as a model of developing epithelium, here we report that Netrin-4 is abundantly expressed in vascular endothelial cells and pancreatic ductal cells, and supports epithelial cell adhesion through integrins α2β1 and α3β1. Interestingly, we find that Netrin-4 recognition by embryonic pancreatic cells through integrins α2β1 and α3β1 promotes insulin and glucagon gene expression. In addition, full genome microarray analysis revealed that fetal pancreatic cell adhesion to Netrin-4 causes a prominent down-regulation of cyclins and up-regulation of negative regulators of the cell cycle. Consistent with these results, a number of other genes whose activities have been linked to developmental decisions and/or cellular differentiation are up-regulated.
Conclusions/significance: Given the recognized function of blood vessels in epithelial tissue morphogenesis, our results provide a mechanism by which endothelial-derived Netrin-4 may function as a pro-differentiation cue for adjacent developing pancreatic cell populations expressing adhesion receptors α2β1 and α3β1 integrins.
Conflict of interest statement
Figures



References
-
- Streuli C. Extracellular matrix remodelling and cellular differentiation. Curr Opin Cell Biol. 1999;11:634–640. - PubMed
-
- Giancotti FG, Ruoslahti E. Integrin signaling. Science. 1999;285:1028–1032. - PubMed
-
- Ruoslahti E, Yamaguchi Y, Hildebrand A, Border WA. Extracellular matrix/growth factor interactions. Cold Spring Harb Symp Quant Biol. 1992;57:309–315. - PubMed
-
- Edlund H. Pancreas: how to get there from the gut? Curr Opin Cell Biol. 1999;11:663–668. - PubMed
-
- Cleaver O, Krieg PA. Notochord patterning of the endoderm. Dev Biol. 2001;234:1–12. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DK055183/DK/NIDDK NIH HHS/United States
- R01 HL075270/HL/NHLBI NIH HHS/United States
- R24 DK080506/DK/NIDDK NIH HHS/United States
- RR04050/RR/NCRR NIH HHS/United States
- R01 DK063443/DK/NIDDK NIH HHS/United States
- P30 DK063491/DK/NIDDK NIH HHS/United States
- DK55183/DK/NIDDK NIH HHS/United States
- DK063491/DK/NIDDK NIH HHS/United States
- DK63443/DK/NIDDK NIH HHS/United States
- DK55183-S1/DK/NIDDK NIH HHS/United States
- DK080506/DK/NIDDK NIH HHS/United States
- R01 CA069112/CA/NCI NIH HHS/United States
- HL075270/HL/NHLBI NIH HHS/United States
- CA69112/CA/NCI NIH HHS/United States
- P41 RR004050/RR/NCRR NIH HHS/United States
- HL62477/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

