Rab18 dynamics in adipocytes in relation to lipogenesis, lipolysis and obesity
- PMID: 21829560
- PMCID: PMC3145781
- DOI: 10.1371/journal.pone.0022931
Rab18 dynamics in adipocytes in relation to lipogenesis, lipolysis and obesity
Abstract
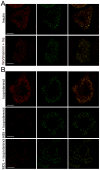
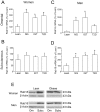
Lipid droplets (LDs) are organelles that coordinate lipid storage and mobilization, both processes being especially important in cells specialized in managing fat, the adipocytes. Proteomic analyses of LDs have consistently identified the small GTPase Rab18 as a component of the LD coat. However, the specific contribution of Rab18 to adipocyte function remains to be elucidated. Herein, we have analyzed Rab18 expression, intracellular localization and function in relation to the metabolic status of adipocytes. We show that Rab18 production increases during adipogenic differentiation of 3T3-L1 cells. In addition, our data show that insulin induces, via phosphatidylinositol 3-kinase (PI3K), the recruitment of Rab18 to the surface of LDs. Furthermore, Rab18 overexpression increased basal lipogenesis and Rab18 silencing impaired the lipogenic response to insulin, thereby suggesting that this GTPase promotes fat accumulation in adipocytes. On the other hand, studies of the β-adrenergic receptor agonist isoproterenol confirmed and extended previous evidence for the participation of Rab18 in lipolysis. Together, our data support the view that Rab18 is a common mediator of lipolysis and lipogenesis and suggests that the endoplasmic reticulum (ER) is the link that enables Rab18 action on these two processes. Finally, we describe, for the first time, the presence of Rab18 in human adipose tissue, wherein the expression of this GTPase exhibits sex- and depot-specific differences and is correlated to obesity. Taken together, these findings indicate that Rab18 is involved in insulin-mediated lipogenesis, as well as in β-adrenergic-induced lipolysis, likely facilitating interaction of LDs with ER membranes and the exchange of lipids between these compartments. A role for Rab18 in the regulation of adipocyte biology under both normal and pathological conditions is proposed.
Conflict of interest statement
Figures






Similar articles
-
Nutritional, hormonal, and depot-dependent regulation of the expression of the small GTPase Rab18 in rodent adipose tissue.J Mol Endocrinol. 2012 Dec 31;50(1):19-29. doi: 10.1530/JME-12-0140. Print 2013 Feb. J Mol Endocrinol. 2012. PMID: 23093555
-
Regulated localization of Rab18 to lipid droplets: effects of lipolytic stimulation and inhibition of lipid droplet catabolism.J Biol Chem. 2005 Dec 23;280(51):42325-35. doi: 10.1074/jbc.M506651200. Epub 2005 Oct 5. J Biol Chem. 2005. PMID: 16207721
-
Rab18 Drift in Lipid Droplet and Endoplasmic Reticulum Interactions of Adipocytes under Obesogenic Conditions.Int J Mol Sci. 2023 Dec 6;24(24):17177. doi: 10.3390/ijms242417177. Int J Mol Sci. 2023. PMID: 38139006 Free PMC article.
-
Rab proteins as regulators of lipid droplet formation and lipolysis.Cell Biol Int. 2016 Oct;40(10):1026-32. doi: 10.1002/cbin.10650. Epub 2016 Sep 5. Cell Biol Int. 2016. PMID: 27453349 Review.
-
Rab18: new insights into the function of an essential protein.Cell Mol Life Sci. 2019 May;76(10):1935-1945. doi: 10.1007/s00018-019-03050-3. Epub 2019 Mar 4. Cell Mol Life Sci. 2019. PMID: 30830238 Free PMC article. Review.
Cited by
-
Spirulina supplement and exercise training affect lipid droplets-related genes expression in visceral adipose tissue.Avicenna J Phytomed. 2024 Jan-Feb;14(1):100-111. doi: 10.22038/AJP.2023.22915. Avicenna J Phytomed. 2024. PMID: 38948175 Free PMC article.
-
Time to Consider the "Exposome Hypothesis" in the Development of the Obesity Pandemic.Nutrients. 2022 Apr 12;14(8):1597. doi: 10.3390/nu14081597. Nutrients. 2022. PMID: 35458158 Free PMC article. Review.
-
Dermatopontin Influences the Development of Obesity-Associated Colon Cancer by Changes in the Expression of Extracellular Matrix Proteins.Int J Mol Sci. 2022 Aug 17;23(16):9222. doi: 10.3390/ijms23169222. Int J Mol Sci. 2022. PMID: 36012487 Free PMC article.
-
The genome of the naturally evolved obesity-prone Ossabaw miniature pig.iScience. 2021 Sep 3;24(9):103081. doi: 10.1016/j.isci.2021.103081. eCollection 2021 Sep 24. iScience. 2021. PMID: 34585119 Free PMC article.
-
Roles of lipid droplets and related proteins in metabolic diseases.Lipids Health Dis. 2024 Jul 19;23(1):218. doi: 10.1186/s12944-024-02212-y. Lipids Health Dis. 2024. PMID: 39030618 Free PMC article. Review.
References
-
- Langin D. Adipose tissue lipolysis as a metabolic pathway to define pharmacological strategies against obesity and the metabolic syndrome. Pharmacol Res. 2006;53:482–491. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

