Probiotics modulate intestinal expression of nuclear receptor and provide counter-regulatory signals to inflammation-driven adipose tissue activation
- PMID: 21829567
- PMCID: PMC3146529
- DOI: 10.1371/journal.pone.0022978
Probiotics modulate intestinal expression of nuclear receptor and provide counter-regulatory signals to inflammation-driven adipose tissue activation
Abstract
Background: Adipocytes from mesenteric white adipose tissue amplify the inflammatory response and participate in inflammation-driven immune dysfunction in Crohn's disease by releasing proinflammatory mediators. Peroxisome proliferator-activated receptors (PPAR)-α and -γ, pregnane x receptor (PXR), farnesoid x receptor (FXR) and liver x-receptor (LXR) are ligand-activated nuclear receptor that provide counter-regulatory signals to dysregulated immunity and modulates adipose tissue.
Aims: To investigate the expression and function of nuclear receptors in intestinal and adipose tissues in a rodent model of colitis and mesenteric fat from Crohn's patients and to investigate their modulation by probiotics.
Methods: Colitis was induced by TNBS administration. Mice were administered vehicle or VSL#3, daily for 10 days. Abdominal fat explants obtained at surgery from five Crohn's disease patients and five patients with colon cancer were cultured with VSL#3 medium.
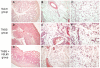
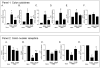
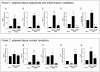
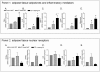
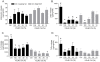
Results: Probiotic administration attenuated development of signs and symptoms of colitis, reduced colonic expression of TNFα, IL-6 and IFNγ and reserved colonic downregulation of PPARγ, PXR and FXR caused by TNBS. Mesenteric fat depots isolated from TNBS-treated animals had increased expression of inflammatory mediators along with PPARγ, FXR, leptin and adiponectin. These changes were prevented by VSL#3. Creeping fat and mesenteric adipose tissue from Crohn's patients showed a differential expression of PPARγ and FXR with both tissue expressing high levels of leptin. Exposure of these tissues to VSL#3 medium abrogates leptin release.
Conclusions: Mesenteric adipose tissue from rodent colitis and Crohn's disease is metabolically active and shows inflammation-driven regulation of PPARγ, FXR and leptin. Probiotics correct the inflammation-driven metabolic dysfunction.
Conflict of interest statement
Figures


References
-
- Sheehan AL, Warren BF, Gear MW, Shepherd NA. Fat-wrapping in Crohn's disease: pathological basis and relevance to surgical practice. Br J Surg. 1992;79:955–958. - PubMed
-
- Smedh K, Olaison G, Nyström PO, Sjödahl R. Intraoperative enteroscopy in Crohn's disease. Br J Surg. 1993;80:897–900. - PubMed
-
- Borley NR, Mortensen NJ, Jewell DP, Warren BF. The relationship between inflammatory and serosal connective tissue changes in ileal Crohn's disease: evidence for a possible causative link. J Pathol. 2000;190:196–202. - PubMed
-
- Fazio VW, Jones IT. Standard surgical treatment of Crohn's disease of the small intestine and ileocolitis. In: Lee ECG, Nolan DJ, eds. Surgery of inflammatory bowel disorders. Edinburgh: Churchill Livingstone. 1987;147–56
-
- Paul G, Schäffler A, Neumeier M, Fürst A, Bataillle F, et al. Profiling adipocytokine secretion from creeping fat in Crohn's disease. Inflamm Bowel Dis. 2006;12:471–477. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

