Disease severity and progression in progressive supranuclear palsy and multiple system atrophy: validation of the NNIPPS--Parkinson Plus Scale
- PMID: 21829612
- PMCID: PMC3150329
- DOI: 10.1371/journal.pone.0022293
Disease severity and progression in progressive supranuclear palsy and multiple system atrophy: validation of the NNIPPS--Parkinson Plus Scale
Abstract
Background: The Natural History and Neuroprotection in Parkinson Plus Syndromes (NNIPPS) study was a large phase III randomized placebo-controlled trial of riluzole in Progressive Supranuclear Palsy (PSP, n = 362) and Multiple System Atrophy (MSA, n = 398). To assess disease severity and progression, we constructed and validated a new clinical rating scale as an ancillary study.
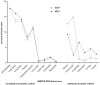
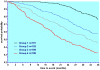
Methods and findings: Patients were assessed at entry and 6-montly for up to 3 years. Evaluation of the scale's psychometric properties included reliability (n = 116), validity (n = 760), and responsiveness (n = 642). Among the 85 items of the initial scale, factor analysis revealed 83 items contributing to 15 clinically relevant dimensions, including Activity of daily Living/Mobility, Axial bradykinesia, Limb bradykinesia, Rigidity, Oculomotor, Cerebellar, Bulbar/Pseudo-bulbar, Mental, Orthostatic, Urinary, Limb dystonia, Axial dystonia, Pyramidal, Myoclonus and Tremor. All but the Pyramidal dimension demonstrated good internal consistency (Cronbach α ≥ 0.70). Inter-rater reliability was high for the total score (Intra-class coefficient = 0.94) and 9 dimensions (Intra-class coefficient = 0.80-0.93), and moderate (Intra-class coefficient = 0.54-0.77) for 6. Correlations of the total score with other clinical measures of severity were good (rho ≥ 0.70). The total score was significantly and linearly related to survival (p<0.0001). Responsiveness expressed as the Standardized Response Mean was high for the total score slope of change (SRM = 1.10), though higher in PSP (SRM = 1.25) than in MSA (SRM = 1.0), indicating a more rapid progression of PSP. The slope of change was constant with increasing disease severity demonstrating good linearity of the scale throughout disease stages. Although MSA and PSP differed quantitatively on the total score at entry and on rate of progression, the relative contribution of clinical dimensions to overall severity and progression was similar.
Conclusions: The NNIPPS-PPS has suitable validity, is reliable and sensitive, and therefore is appropriate for use in clinical studies with PSP or MSA.
Trial registration: ClinicalTrials.gov NCT00211224.
Conflict of interest statement
Figures

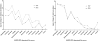
References
-
- Litvan I, Bhatia KP, Burn DJ, Goetz CG, Lang AE, et al. Movement Disorders Society Scientific Issues Committee report: SIC Task Force appraisal of clinical diagnostic criteria for Parkinsonian disorders. Mov Disord. 2003;18:467–486. - PubMed
-
- Testa D, Monza D, Ferrarini M, Soliveri P, Girotti F, et al. Comparison of natural histories of progressive supranuclear palsy and multiple system atrophy. Neurol Sci. 2001;22:247–251. - PubMed
-
- Ben-Shlomo Y, Wenning GK, Tison F, Quinn NP. Survival of patients with pathologically proven multiple system atrophy: a meta-analysis. Neurology. 1997;48:384–93. - PubMed
-
- Golbe LI, Ohman-Strickland PA. A clinical rating scale for progressive supranuclear palsy. Brain. 2007;130:1552–1565. - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

