ISL1 promotes pancreatic islet cell proliferation
- PMID: 21829621
- PMCID: PMC3150357
- DOI: 10.1371/journal.pone.0022387
ISL1 promotes pancreatic islet cell proliferation
Abstract
Background: Islet 1 (ISL1), a LIM-homeodomain transcription factor is essential for promoting pancreatic islets proliferation and maintaining endocrine cells survival in embryonic and postnatal pancreatic islets. However, how ISL1 exerts the role in adult islets is, to date, not clear.
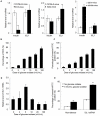
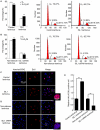
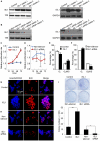
Methodology/principal findings: Our results show that ISL1 expression was up-regulated at the mRNA level both in cultured pancreatic cells undergoing glucose oxidase stimulation as well in type 1 and type 2 diabetes mouse models. The knockdown of ISL1 expression increased the apoptosis level of HIT-T15 pancreatic islet cells. Using HIT-T15 and primary adult islet cells as cell models, we show that ISL1 promoted adult pancreatic islet cell proliferation with increased c-Myc and CyclinD1 transcription, while knockdown of ISL1 increased the proportion of cells in G(1) phase and decreased the proportion of cells in G(2)/M and S phases. Further investigation shows that ISL1 activated both c-Myc and CyclinD1 transcription through direct binding on their promoters.
Conclusions/significance: ISL1 promoted adult pancreatic islet cell proliferation and probably by activating c-Myc and CyclinD1 transcription through direct binding on their promoters. Our findings extend the knowledge about the crucial role of ISL1 in maintaining mature islet cells homeostasis. Our results also provide insights into the new regulation relationships between ISL1 and other growth factors.
Conflict of interest statement
Figures
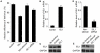


Similar articles
-
ISL1, a novel regulator of CCNB1, CCNB2 and c-MYC genes, promotes gastric cancer cell proliferation and tumor growth.Oncotarget. 2016 Jun 14;7(24):36489-36500. doi: 10.18632/oncotarget.9269. Oncotarget. 2016. PMID: 27183908 Free PMC article.
-
The islet-expressed Lhx1 transcription factor interacts with Islet-1 and contributes to glucose homeostasis.Am J Physiol Endocrinol Metab. 2019 Mar 1;316(3):E397-E409. doi: 10.1152/ajpendo.00235.2018. Epub 2019 Jan 8. Am J Physiol Endocrinol Metab. 2019. PMID: 30620636 Free PMC article.
-
The SSBP3 co-regulator is required for glucose homeostasis, pancreatic islet architecture, and beta-cell identity.Mol Metab. 2023 Oct;76:101785. doi: 10.1016/j.molmet.2023.101785. Epub 2023 Aug 1. Mol Metab. 2023. PMID: 37536498 Free PMC article.
-
Expression and function of the LIM-homeodomain transcription factor Islet-1 in the developing and mature vertebrate retina.Exp Eye Res. 2015 Sep;138:22-31. doi: 10.1016/j.exer.2015.06.021. Epub 2015 Jun 26. Exp Eye Res. 2015. PMID: 26122047 Review.
-
Oncogenic co-operation in beta-cell tumorigenesis.Endocr Relat Cancer. 2001 Dec;8(4):307-14. doi: 10.1677/erc.0.0080307. Endocr Relat Cancer. 2001. PMID: 11733227 Review.
Cited by
-
microRNA-9 and -29a regulate the progression of diabetic peripheral neuropathy via ISL1-mediated sonic hedgehog signaling pathway.Aging (Albany NY). 2020 Jun 16;12(12):11446-11465. doi: 10.18632/aging.103230. Epub 2020 Jun 16. Aging (Albany NY). 2020. PMID: 32544883 Free PMC article.
-
Wnt-promoted Isl1 expression through a novel TCF/LEF1 binding site and H3K9 acetylation in early stages of cardiomyocyte differentiation of P19CL6 cells.Mol Cell Biochem. 2014 Jun;391(1-2):183-92. doi: 10.1007/s11010-014-2001-y. Epub 2014 Mar 8. Mol Cell Biochem. 2014. PMID: 24610003
-
Skin fibroblasts from patients with type 1 diabetes (T1D) can be chemically transdifferentiated into insulin-expressing clusters: a transgene-free approach.PLoS One. 2014 Jun 25;9(6):e100369. doi: 10.1371/journal.pone.0100369. eCollection 2014. PLoS One. 2014. PMID: 24963634 Free PMC article.
-
Long non-coding RNA LINC00673 silencing inhibits proliferation and drug resistance of prostate cancer cells via decreasing KLF4 promoter methylation.J Cell Mol Med. 2020 Jan;24(2):1878-1892. doi: 10.1111/jcmm.14883. Epub 2019 Dec 27. J Cell Mol Med. 2020. PMID: 31881124 Free PMC article.
-
G6PD promotes renal cell carcinoma proliferation through positive feedback regulation of p-STAT3.Oncotarget. 2017 Nov 20;8(65):109043-109060. doi: 10.18632/oncotarget.22566. eCollection 2017 Dec 12. Oncotarget. 2017. PMID: 29312589 Free PMC article.
References
-
- Habener JF, Kemp DM, Thomas MK. Minireview: transcriptional regulation in pancreatic development. Endocrinology. 2005;146:1025–1034. - PubMed
-
- Lyttle BM, Li J, Krishnamurthy M, Fellows F, Wheeler MB, et al. Transcription factor expression in the developing human fetal endocrine pancreas. Diabetologia. 2008;51:1169–1180. - PubMed
-
- Bonner-Weir S. Islet growth and development in the adult. J Mol Endocrinol. 2000;24:297–302. - PubMed
-
- Dor Y, Brown J, Martinez OI, Melton DA. Adult pancreatic beta-cells are formed by self-duplication rather than stem-cell differentiation. Nature. 2004;429:41–46. - PubMed
-
- Karlsson O, Thor S, Norberg T, Ohlsson H, Edlund T. Insulin gene enhancer binding protein Isl-1 is a member of a novel class of proteins containing both a homeo- and a Cys-His domain. Nature. 1990;344:879–882. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

