Addition of bevacizumab to chemotherapy in advanced non-small cell lung cancer: a systematic review and meta-analysis
- PMID: 21829644
- PMCID: PMC3149053
- DOI: 10.1371/journal.pone.0022681
Addition of bevacizumab to chemotherapy in advanced non-small cell lung cancer: a systematic review and meta-analysis
Abstract
Introduction: Recently, studies have demonstrated that the addition of bevacizumab to chemotherapy could be associated with better outcomes in patients with advanced non-small cell lung cancer (NSCLC). However, the benefit seems to be dependent on the drugs used in the chemotherapy regimens. This systematic review evaluated the strength of data on efficacy of the addition of bevacizumab to chemotherapy in advanced NSCLC.
Methods: PubMed, EMBASE, and Cochrane databases were searched. Eligible studies were randomized clinical trials (RCTs) that evaluated chemotherapy with or without bevacizumab in patients with advanced NSCLC. The outcomes included overall survival (OS), progression-free survival (PFS), response rate (RR), toxicities and treatment related mortality. Hazard ratios (HR) and odds ratios (OR) were used for the meta-analysis and were expressed with 95% confidence intervals (CI).
Results: We included results reported from five RCTs, with a total of 2,252 patients included in the primary analysis, all of them using platinum-based chemotherapy regimens. Compared to chemotherapy alone, the addition of bevacizumab to chemotherapy resulted in a significant longer OS (HR 0.89; 95% CI 0.79 to 0.99; p = 0.04), longer PFS (HR 0.73; 95% CI 0.66 to 0.82; p<0.00001) and higher response rates (OR 2.34; 95% CI 1.89 to 2.89; p<0.00001). We found no heterogeneity between trials, in all comparisons. There was a slight increase in toxicities in bevacizumab group, as well as an increased rate of treatment-related mortality.
Conclusions: The addition of bevacizumab to chemotherapy in patients with advanced NSCLC prolongs OS, PFS and RR. Considering the toxicities added, and the small absolute benefits found, bevacizumab plus platinum-based chemotherapy can be considered an option in selected patients with advanced NSCLC. However, risks and benefits should be discussed with patients before decision making.
Conflict of interest statement
Figures

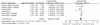

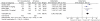

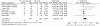
References
-
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, et al. Cancer statistics, 2009. CA Cancer J Clin. 2009;59(4):225–49. - PubMed
-
- Chen F, Cole P, Bina WF. Time trend and geographic patterns of lung adenocarcinoma in the United States, 1973–2002. Cancer Epidemiol Biomarkers Prev. 2007;16(12):2724–9. - PubMed
-
- Schiller JH, Harrington D, Belani CP, Langer C, Sandler A, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med. 2002;346(2):92–8. - PubMed
-
- Yuan A, Yu CJ, Kuo SH, Chen WJ, Lin FY, et al. Vascular endothelial growth factor 189 mRNA isoform expression specifically correlates with tumor angiogenesis, patient survival, and postoperative relapse in non-small-cell lung cancer. J Clin Oncol. 2001;19(2):432–41. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

