Generation of a cell culture-adapted hepatitis C virus with longer half life at physiological temperature
- PMID: 21829654
- PMCID: PMC3150383
- DOI: 10.1371/journal.pone.0022808
Generation of a cell culture-adapted hepatitis C virus with longer half life at physiological temperature
Abstract
Background: We previously reported infectious HCV clones that contain the convenient reporters, green fluorescent protein (GFP) and Renilla luciferase (Rluc), in the NS5a-coding sequence. Although these viruses were useful in monitoring viral proliferation and screening of anti-HCV drugs, the infectivity and yield of the viruses were low.
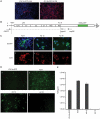
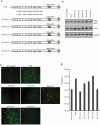
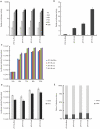
Methodology/principal findings: In order to obtain a highly efficient HCV cultivation system, we transfected Huh7.5.1 cells [1] with JFH 5a-GFP RNA and then cultivated cells for 20 days. We found a highly infectious HCV clone containing two cell culture-adapted mutations. Two cell culture-adapted mutations which were responsible for the increased viral infectivity were located in E2 and p7 protein coding regions. The viral titer of the variant was ∼100-fold higher than that of the parental virus. The mutation in the E2 protein increased the viability of virus at 37°C by acquiring prolonged interaction capability with a HCV receptor CD81. The wild-type and p7-mutated virus had a half-life of ∼2.5 to 3 hours at 37°C. In contrast, the half-life of viruses, which contained E2 mutation singly and combination with the p7 mutation, was 5 to 6 hours at 37°C. The mutation in the p7 protein, either singly or in combination with the E2 mutation, enhanced infectious virus production about 10-50-fold by facilitating an early step of virion production.
Conclusion/significance: The mutation in the E2 protein generated by the culture system increases virion viability at 37°C. The adaptive mutation in the p7 protein facilitates an earlier stage of virus production, such as virus assembly and/or morphogenesis. These reporter-containing HCV viruses harboring adaptive mutations are useful in investigations of the viral life cycle and for developing anti-viral agents against HCV.
Conflict of interest statement
Figures


Similar articles
-
Cooperation between the Hepatitis C Virus p7 and NS5B Proteins Enhances Virion Infectivity.J Virol. 2015 Nov;89(22):11523-33. doi: 10.1128/JVI.01185-15. Epub 2015 Sep 9. J Virol. 2015. PMID: 26355084 Free PMC article.
-
Adaptive Mutations Enhance Assembly and Cell-to-Cell Transmission of a High-Titer Hepatitis C Virus Genotype 5a Core-NS2 JFH1-Based Recombinant.J Virol. 2015 Aug;89(15):7758-75. doi: 10.1128/JVI.00039-15. Epub 2015 May 20. J Virol. 2015. PMID: 25995244 Free PMC article.
-
A cell culture adapted HCV JFH1 variant that increases viral titers and permits the production of high titer infectious chimeric reporter viruses.PLoS One. 2012;7(9):e44965. doi: 10.1371/journal.pone.0044965. Epub 2012 Sep 13. PLoS One. 2012. PMID: 23028707 Free PMC article.
-
Efficient infectious cell culture systems of the hepatitis C virus (HCV) prototype strains HCV-1 and H77.J Virol. 2015 Jan;89(1):811-23. doi: 10.1128/JVI.02877-14. Epub 2014 Oct 29. J Virol. 2015. PMID: 25355880 Free PMC article.
-
The elusive function of the hepatitis C virus p7 protein.Virology. 2014 Aug;462-463:377-87. doi: 10.1016/j.virol.2014.04.018. Epub 2014 Jul 4. Virology. 2014. PMID: 25001174 Free PMC article. Review.
Cited by
-
The p7 protein of the hepatitis C virus induces cell death differently from the influenza A virus viroporin M2.Virus Res. 2013 Mar;172(1-2):24-34. doi: 10.1016/j.virusres.2012.12.005. Epub 2012 Dec 12. Virus Res. 2013. PMID: 23246447 Free PMC article.
-
Hepatitis C virus epitope exposure and neutralization by antibodies is affected by time and temperature.Virology. 2012 Jan 20;422(2):174-84. doi: 10.1016/j.virol.2011.10.023. Epub 2011 Nov 12. Virology. 2012. PMID: 22078164 Free PMC article.
-
RACK1 mediates rewiring of intracellular networks induced by hepatitis C virus infection.PLoS Pathog. 2019 Sep 16;15(9):e1008021. doi: 10.1371/journal.ppat.1008021. eCollection 2019 Sep. PLoS Pathog. 2019. PMID: 31525236 Free PMC article.
-
Cooperation between the Hepatitis C Virus p7 and NS5B Proteins Enhances Virion Infectivity.J Virol. 2015 Nov;89(22):11523-33. doi: 10.1128/JVI.01185-15. Epub 2015 Sep 9. J Virol. 2015. PMID: 26355084 Free PMC article.
-
A Library of Infectious Hepatitis C Viruses with Engineered Mutations in the E2 Gene Reveals Growth-Adaptive Mutations That Modulate Interactions with Scavenger Receptor Class B Type I.J Virol. 2016 Nov 14;90(23):10499-10512. doi: 10.1128/JVI.01011-16. Print 2016 Dec 1. J Virol. 2016. PMID: 27630236 Free PMC article.
References
-
- Liang TJ, Jeffers LJ, Reddy KR, De Medina M, Parker IT, et al. Viral pathogenesis of hepatocellular carcinoma in the United States. Hepatology. 1993;18:1326–1333. - PubMed
-
- Wasley A, Alter MJ. Epidemiology of hepatitis C: geographic differences and temporal trends. Semin Liver Dis. 2000;20:1–16. - PubMed
-
- Fried MW, Shiffman ML, Reddy KR, Smith C, Marinos G, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347:975–982. - PubMed
-
- Dienstag JL, McHutchison JG. American Gastroenterological Association technical review on the management of hepatitis C. Gastroenterology. 2006;130:231–264; quiz 214–237. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

