Zona pellucida domain-containing protein β-tectorin is crucial for zebrafish proper inner ear development
- PMID: 21829695
- PMCID: PMC3149068
- DOI: 10.1371/journal.pone.0023078
Zona pellucida domain-containing protein β-tectorin is crucial for zebrafish proper inner ear development
Erratum in
- PLoS One. 2012;7(2). doi: 10.1371/annotation/7f7eb48c-7429-4119-89de-9c9bc3d85f04
Abstract
Background: The zona pellucida (ZP) domain is part of many extracellular proteins with diverse functions from structural components to receptors. The mammalian β-tectorin is a protein of 336 amino acid residues containing a single ZP domain and a putative signal peptide at the N-terminus of the protein. It is 1 component of a gel-like structure called the tectorial membrane which is involved in transforming sound waves into neuronal signals and is important for normal auditory function. β-Tectorin is specifically expressed in the mammalian and avian inner ear.
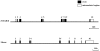
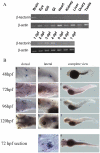
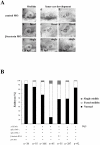
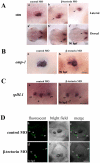
Methodology/principal findings: We identified and cloned the gene encoding zebrafish β-tectorin. Through whole-mount in situ hybridization, we demonstrated that β-tectorin messenger RNA was expressed in the otic placode and specialized sensory patch of the inner ear during zebrafish embryonic stages. Morpholino knockdown of zebrafish β-tectorin affected the position and number of otoliths in the ears of morphants. Finally, swimming behaviors of β-tectorin morphants were abnormal since the development of the inner ear was compromised.
Conclusions/significance: Our results reveal that zebrafish β-tectorin is specifically expressed in the zebrafish inner ear, and is important for regulating the development of the zebrafish inner ear. Lack of zebrafish β-tectorin caused severe defects in inner ear formation of otoliths and function.
Conflict of interest statement
Figures


References
-
- Jovine L, Darie CC, Litscher ES, Wassarman PM. Zona pellucida domain proteins. Annu Rev Biochem. 2005/06/15 ed. 2005:83–114. - PubMed
-
- Jovine L, Qi H, Williams Z, Litscher E, Wassarman PM. The ZP domain is a conserved module for polymerization of extracellular proteins. Nat Cell Biol. 2002;4:457–461. - PubMed
-
- Wassarman PM, Jovine L, Litscher ES. A profile of fertilization in mammals. Nat Cell Biol. 2001;3:E59–64. - PubMed
-
- Bork P, Sander C. A large domain common to sperm receptors (Zp2 and Zp3) and TGF-beta type III receptor. FEBS Lett. 1992;300:237–240. - PubMed
-
- Verhoeven K, Van Laer L, Kirschhofer K, Legan PK, Hughes DC, et al. Mutations in the human alpha-tectorin gene cause autosomal dominant non-syndromic hearing impairment. Nat Genet. 1998;19:60–62. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

