Retinal input influences the size and corticocortical connectivity of visual cortex during postnatal development in the ferret
- PMID: 21830218
- PMCID: PMC3670942
- DOI: 10.1002/cne.22738
Retinal input influences the size and corticocortical connectivity of visual cortex during postnatal development in the ferret
Abstract
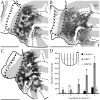
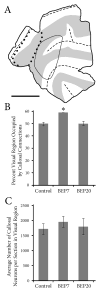
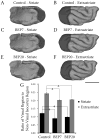
Retinal input plays an important role in the specification of topographically organized circuits and neuronal response properties, but the mechanism and timing of this effect is not known in most species. A system that shows dramatic dependence on retinal influences is the interhemispheric connection through the corpus callosum. Using ferrets, we analyzed the extent to which development of the visual callosal pattern depends on retinal influences, and explored the period during which these influences are required for normal pattern formation. We studied the mature callosal patterns in normal ferrets and in ferrets bilaterally enucleated (BE) at postnatal day 7 (P7) or P20. Callosal patterns were revealed in tangential sections from unfolded and flattened brains following multiple injections of horseradish peroxidase in the opposite hemisphere. We also estimated the effect of enucleation on the surface areas of striate and extrastriate visual cortex by using magnetic resonance imaging (MRI) data from intact brains. In BEP7 ferrets we found that the pattern of callosal connections was highly anomalous and the sizes of both striate and extrastriate visual cortex were significantly reduced. In contrast, enucleation at P20 had no significant effect on the callosal pattern, but it still caused a reduction in the size of striate and extrastriate visual cortex. Finally, retinal deafferentation had no significant effect on the number of visual callosal neurons. These results indicate that the critical period during which the eyes influence the development of callosal patterns, but not the size of visual cortex, ends by P20 in the ferret.
Copyright © 2011 Wiley Periodicals, Inc.
Figures
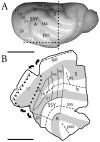
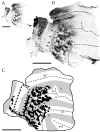

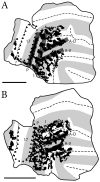

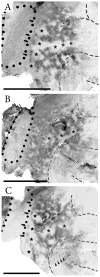
Similar articles
-
Role of retinal input on the development of striate-extrastriate patterns of connections in the rat.J Comp Neurol. 2012 Oct 1;520(14):3256-76. doi: 10.1002/cne.23096. J Comp Neurol. 2012. PMID: 22430936 Free PMC article.
-
Overall pattern of callosal connections in visual cortex of normal and enucleated cats.J Comp Neurol. 1995 Dec 11;363(2):161-76. doi: 10.1002/cne.903630202. J Comp Neurol. 1995. PMID: 8642068
-
Retinal influences specify cortico-cortical maps by postnatal day six in rats and mice.J Comp Neurol. 2003 Apr 28;459(2):156-72. doi: 10.1002/cne.10615. J Comp Neurol. 2003. PMID: 12640667
-
Deafferentation-induced plasticity of visual callosal connections: predicting critical periods and analyzing cortical abnormalities using diffusion tensor imaging.Neural Plast. 2012;2012:250196. doi: 10.1155/2012/250196. Epub 2012 Nov 8. Neural Plast. 2012. PMID: 23213572 Free PMC article. Review.
-
The visual callosal connection: a connection like any other?Neural Plast. 2013;2013:397176. doi: 10.1155/2013/397176. Epub 2013 Mar 24. Neural Plast. 2013. PMID: 23634306 Free PMC article. Review.
Cited by
-
Reorganization of Visual Callosal Connections Following Alterations of Retinal Input and Brain Damage.Front Syst Neurosci. 2016 Nov 14;10:86. doi: 10.3389/fnsys.2016.00086. eCollection 2016. Front Syst Neurosci. 2016. PMID: 27895559 Free PMC article. Review.
-
Reorganization of thalamocortical connections in congenitally blind humans.Hum Brain Mapp. 2023 Apr 1;44(5):2039-2049. doi: 10.1002/hbm.26192. Epub 2023 Jan 20. Hum Brain Mapp. 2023. PMID: 36661404 Free PMC article.
-
Impact of Early and Late Visual Deprivation on the Structure of the Corpus Callosum: A Study Combining Thickness Profile with Surface Tensor-Based Morphometry.Neuroinformatics. 2015 Jul;13(3):321-336. doi: 10.1007/s12021-014-9259-9. Neuroinformatics. 2015. PMID: 25649876 Free PMC article.
-
Neural pathways conveying novisual information to the visual cortex.Neural Plast. 2013;2013:864920. doi: 10.1155/2013/864920. Epub 2013 Jun 6. Neural Plast. 2013. PMID: 23840972 Free PMC article. Review.
-
Aberrant development of excitatory circuits to inhibitory neurons in the primary visual cortex after neonatal binocular enucleation.Sci Rep. 2021 Feb 4;11(1):3163. doi: 10.1038/s41598-021-82679-2. Sci Rep. 2021. PMID: 33542365 Free PMC article.
References
-
- Abel PL, O’Brien BJ, Olavarria JF. Organization of callosal linkages in visual area V2 of macaque monkey. J Comp Neurol. 2000;428:278–293. - PubMed
-
- Aggoun-Aouaoui D, Kiper DC, Innocenti GM. Growth of callosal terminal arbors in primary visual areas of the cat. Eur J Neurosci. 1996;8:1132–1148. - PubMed
-
- Bock AS, Kroenke CD, Taber EN, Olavarria JF. Critical period for the effects of neonatal enucleation on callosal connectivity and water diffusion anisotropy in the ferret visual system. Program No. 580.16; Society for Neuroscience Annual Meeting; 2010; San Diego, CA. 2010a.
-
- Bourdet C, Olavarria JF, Van Sluyters RC. Distribution of visual callosal neurons in normal and strabismic cats. J Comp Neurol. 1996;366:259–269. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

