Structural classification and properties of ketoacyl synthases
- PMID: 21830247
- PMCID: PMC3218358
- DOI: 10.1002/pro.712
Structural classification and properties of ketoacyl synthases
Abstract
Ketoacyl synthases (KSs) catalyze condensing reactions combining acyl-CoA or acyl-acyl carrier protein (acyl-ACP) with malonyl-CoA to form 3-ketoacyl-CoA or with malonyl-ACP to form 3-ketoacyl-ACP. In each case, the resulting acyl chain is two carbon atoms longer than before, and CO2 and either CoA or ACP are formed. KSs also join other activated molecules in the polyketide synthesis cycle. Our classification of KSs by their primary and tertiary structures instead of by their substrates and the reactions that they catalyze enhances insights into this enzyme group. KSs fall into five families separated by their characteristic primary structures, each having members with the same catalytic residues, mechanisms, and tertiary structures. KS1 members, overwhelmingly named 3-ketoacyl-ACP synthase III or its variants, are produced predominantly by bacteria. Members of KS2 are mainly produced by plants, and they are usually long-chain fatty acid elongases/condensing enzymes and 3-ketoacyl-CoA synthases. KS3, a very large family, is composed of bacterial and eukaryotic 3-ketoacyl-ACP synthases I and II, often found in multidomain fatty acid and polyketide synthases. Most of the chalcone synthases, stilbene synthases, and naringenin-chalcone synthases in KS4 are from eukaryota. KS5 members are all from eukaryota, most are produced by animals, and they are mainly fatty acid elongases. All families except KS3 are split into subfamilies whose members have statistically significant differences in their primary structures. KS1 through KS4 appear to be part of the same clan. KS sequences, tertiary structures, and family classifications are available on the continuously updated ThYme (Thioester-active enzYme) database.
Figures
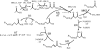
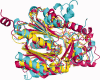
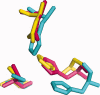
References
-
- Moche M, Dehesh K, Edwards P, Lindqvist Y. The crystal structure of β-ketoacyl-acyl carrier protein synthase II from Synechocystis sp. at 1.54 Å resolution and its relationship to other condensing enzymes. J Mol Biol. 2001;305:491–503. - PubMed
-
- Blacklock BJ, Jaworski JG. Substrate specificity of Arabidopsis 3-ketoacyl-CoA synthases. Biochem Biophys Res Commun. 2006;346:583–590. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

