The yeast ABC transporter Pdr18 (ORF YNR070w) controls plasma membrane sterol composition, playing a role in multidrug resistance
- PMID: 21831043
- PMCID: PMC3215286
- DOI: 10.1042/BJ20110876
The yeast ABC transporter Pdr18 (ORF YNR070w) controls plasma membrane sterol composition, playing a role in multidrug resistance
Abstract
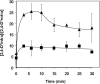
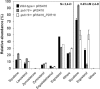
The action of multidrug efflux pumps in MDR (multidrug resistance) acquisition has been proposed to partially depend on the transport of physiological substrates which may indirectly affect drug partition and transport across cell membranes. In the present study, the PDR18 gene [ORF (open reading frame) YNR070w], encoding a putative PDR (pleiotropic drug resistance) transporter of the ATP-binding cassette superfamily, was found to mediate plasma membrane sterol incorporation in yeast. The physiological role of Pdr18 is demonstrated to affect plasma membrane potential and is proposed to underlie its action as a MDR determinant, conferring resistance to the herbicide 2,4-D (2,4-dichlorophenoxyacetic acid). The action of Pdr18 in yeast tolerance to 2,4-D, which was found to contribute to reduce [(14)C]2,4-D intracellular accumulation, may be indirect, given the observation that 2,4-D exposure deeply affects the sterol plasma membrane composition, this effect being much stronger in a Δpdr18 background. PDR18 activation under 2,4-D stress is regulated by the transcription factors Nrg1, controlling carbon source availability and the stress response, and, less significantly, Yap1, involved in oxidative stress and MDR, and Pdr3, a key regulator of the yeast PDR network, consistent with a broad role in stress defence. Taken together, the results of the present study suggest that Pdr18 plays a role in plasma membrane sterol incorporation, this physiological trait contributing to an MDR phenotype.
Figures





References
-
- Roepe P. D., Wei L. Y., Hoffman M. M., Fritz F. Altered drug translocation mediated by the MDR protein: direct, indirect, or both? J. Bioenerg. Biomembr. 1996;28:541–555. - PubMed
-
- Sá-Correia I., Santos S., Teixeira M., Cabrito T., Mira N. Drug:H+ antiporters in chemical stress response in yeast. Trends Microbiol. 2009;17:22–31. - PubMed
-
- Jungwirth H., Kuchler K. Yeast ABC transporters: a tale of sex, stress, drugs and aging. FEBS Lett. 2006;580:1131–1138. - PubMed
-
- Teixeira M. C., Cabrito T. R., Hanif Z. M., Vargas R. C., Tenreiro S., Sá-Correia I. Yeast response and tolerance to polyamine toxicity involving the drug:H+ antiporter Qdr3 and the transcription factors Yap1 and Gcn4. Microbiology. 2011;157:945–956. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

