Perinatal exposure to Δ9-tetrahydrocannabinol triggers profound defects in T cell differentiation and function in fetal and postnatal stages of life, including decreased responsiveness to HIV antigens
- PMID: 21831965
- PMCID: PMC3199982
- DOI: 10.1124/jpet.111.181206
Perinatal exposure to Δ9-tetrahydrocannabinol triggers profound defects in T cell differentiation and function in fetal and postnatal stages of life, including decreased responsiveness to HIV antigens
Abstract
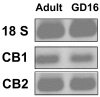
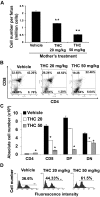
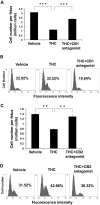
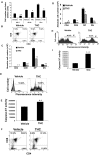
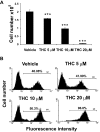
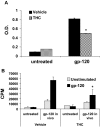
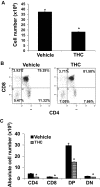
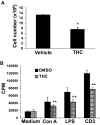
Marijuana abuse is very prominent among pregnant women. Although marijuana cannabinoids have been shown to exert immunosuppression in adults, virtually nothing is known about the effects of marijuana use during pregnancy on the developing immune system of the fetus and during postnatal life. We noted that murine fetal thymus expressed high levels of the cannabinoid receptors CB1 and CB2. Moreover, perinatal exposure to Δ(9)-tetrahydrocannabinol (THC) had a profound effect on the fetus as evidenced by a decrease in thymic cellularity on gestational days 16, 17, and 18 and postgestational day 1 and marked alterations in T cell subpopulations. These outcomes were reversed by CB1/CB2 antagonists, suggesting that THC-mediated these effects through cannabinoid receptors. Thymic atrophy induced in the fetus correlated with caspase-dependent apoptosis in thymocytes. Thymic atrophy was the result of direct action of THC and not based on maternal factors inasmuch as THC was able to induce T cell apoptosis in vitro in fetal thymic organ cultures. It is noteworthy that perinatal exposure to THC also had a profound effect on the immune response during postnatal life. Peripheral T cells from such mice showed decreased proliferative response to T cell mitogen as well as both T cell and antibody response to HIV-1 p17/p24/gp120 antigens. Together, our data demonstrate for the first time that perinatal exposure to THC triggers profound T cell dysfunction, thereby suggesting that the offspring of marijuana abusers who have been exposed to THC in utero may be at a higher risk of exhibiting immune dysfunction and contracting infectious diseases including HIV.
Figures
Similar articles
-
Delta(9)-tetrahydrocannabinol-induced apoptosis in the thymus and spleen as a mechanism of immunosuppression in vitro and in vivo.J Pharmacol Exp Ther. 2002 Aug;302(2):451-65. doi: 10.1124/jpet.102.033506. J Pharmacol Exp Ther. 2002. PMID: 12130702
-
Rimonabant-induced Delta9-tetrahydrocannabinol withdrawal in rhesus monkeys: discriminative stimulus effects and other withdrawal signs.J Pharmacol Exp Ther. 2010 Jul;334(1):347-56. doi: 10.1124/jpet.110.168435. Epub 2010 Apr 7. J Pharmacol Exp Ther. 2010. PMID: 20375197 Free PMC article.
-
Cannabinoid receptors and T helper cells.J Neuroimmunol. 2004 Feb;147(1-2):91-4. doi: 10.1016/j.jneuroim.2003.10.019. J Neuroimmunol. 2004. PMID: 14741435
-
[Cannabis and cannabinoids as drugs].Tidsskr Nor Laegeforen. 2007 Mar 1;127(5):579-82. Tidsskr Nor Laegeforen. 2007. PMID: 17332810 Review. Norwegian.
-
Synaptic targets of Δ9-tetrahydrocannabinol in the central nervous system.Cold Spring Harb Perspect Med. 2013 Aug 1;3(8):a012237. doi: 10.1101/cshperspect.a012237. Cold Spring Harb Perspect Med. 2013. PMID: 23209160 Free PMC article. Review.
Cited by
-
Effects of Cannabinoids on T-cell Function and Resistance to Infection.J Neuroimmune Pharmacol. 2015 Jun;10(2):204-16. doi: 10.1007/s11481-015-9603-3. Epub 2015 Apr 16. J Neuroimmune Pharmacol. 2015. PMID: 25876735 Free PMC article. Review.
-
Cannabinoids' Role in Modulating Central and Peripheral Immunity in Neurodegenerative Diseases.Int J Mol Sci. 2024 Jun 10;25(12):6402. doi: 10.3390/ijms25126402. Int J Mol Sci. 2024. PMID: 38928109 Free PMC article. Review.
-
Δ⁹-Tetrahydrocannabinol attenuates allogeneic host-versus-graft response and delays skin graft rejection through activation of cannabinoid receptor 1 and induction of myeloid-derived suppressor cells.J Leukoc Biol. 2015 Sep;98(3):435-47. doi: 10.1189/jlb.3A0115-030RR. Epub 2015 Jun 1. J Leukoc Biol. 2015. PMID: 26034207 Free PMC article.
-
Gestational exposure to the cannabinoid WIN 55,212-2 and its effect on the innate intestinal immune response.Sci Rep. 2019 Dec 30;9(1):20340. doi: 10.1038/s41598-019-56653-y. Sci Rep. 2019. PMID: 31889093 Free PMC article.
-
Fetal endocannabinoids orchestrate the organization of pancreatic islet microarchitecture.Proc Natl Acad Sci U S A. 2015 Nov 10;112(45):E6185-94. doi: 10.1073/pnas.1519040112. Epub 2015 Oct 22. Proc Natl Acad Sci U S A. 2015. PMID: 26494286 Free PMC article.
References
-
- Azorlosa JL, Heishman SJ, Stitzer ML, Mahaffey JM. (1992) Marijuana smoking: effect of varying Δ9-tetrahydrocannabinol content and number of puffs. J Pharmacol Exp Ther 261:114–122 - PubMed
-
- Beal JE, Olson R, Laubenstein L, Morales JO, Bellman P, Yangco B, Lefkowitz L, Plasse TF, Shepard KV. (1995) Dronabinol as a treatment for anorexia associated with weight loss in patients with AIDS. J Pain Symptom Manage 10:89–97 - PubMed
-
- Berdyshev EV. (2000) Cannabinoid receptors and the regulation of immune response. Chem Phys Lipids 108:169–190 - PubMed
-
- Bluhm EC, Daniels J, Pollock BH, Olshan AF, and Children's Oncology Group (United States) (2006) Maternal use of recreational drugs and neuroblastoma in offspring: a report from the Children's Oncology Group (United States). Cancer Causes Control 17:663–669 - PubMed
-
- Bonnin A, de Miguel R, Hernández ML, Ramos JA, Fernández-Ruiz JJ. (1995) The prenatal exposure to Δ9-tetrahydrocannabinol affects the gene expression and the activity of tyrosine hydroxylase during early brain development. Life Sci 56:2177–2184 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

