A comprehensive evaluation of the proficiency testing program for the HIV-1 BED incidence assay
- PMID: 21832016
- PMCID: PMC3187299
- DOI: 10.1128/JCM.01122-11
A comprehensive evaluation of the proficiency testing program for the HIV-1 BED incidence assay
Abstract
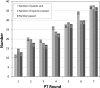
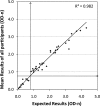
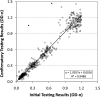
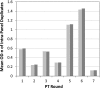
The HIV-1 BED incidence assay was developed at the Centers for Disease Control and Prevention and since 2005 has been available as a commercial kit for use in HIV-1 incidence surveillance. A BED-specific proficiency testing (PT) program was initiated in 2006 that included a panel of eight coded specimens (six unique and two duplicates) to participating laboratories. The number of participating laboratories increased from 12 to 38 from 2006 to 2009. Overall, 96.1% of the laboratories reported results, and 95.4% of those reporting achieved a 100% score. The observed mean normalized optical density (OD-n) values of all participants correlated well with the expected OD-n values for all specimens (R(2) = 0.98) used for seven PT rounds. BED testing demonstrated high reproducibility among all laboratories, with an agreement of 99.3% (574/578) between initial and confirmatory classification and regression statistics of R(2) = 0.96, slope = 1.022, and intercept = 0.0066. Reproducibility among duplicate specimens was very high during each PT round, with mean deviation of 1.8%. Analysis of controls and calibrator specimen for all 343 runs showed a coefficient of variation of ca. 20% for raw ODs in the dynamic range, which was reduced to <10% when the OD was normalized (OD-n). Most laboratories that failed the PT assessment had transcriptional errors, kit reagent problems, or specimen handling errors. Thus, the BED-specific PT program enabled us to track performance of different laboratories conducting the BED assay while identifying areas for improvements. This program will also serve as a template for future PT programs for new incidence assays as they become available.
Figures
References
-
- Cha Y. J., Cho H. I. 2002. External quality assurance in diagnostic immunology: a twenty-year experience in Korea. Southeast Asian J. Trop. Med. Public Health 33(Suppl. 2):104–111 - PubMed
-
- Chalermchan W., Pitak S., Sungkawasee S. 2007. Evaluation of Thailand national external quality assessment on HIV testing. Int. J. Health Care Quality Assurance 20:130–140 - PubMed
-
- Constantine N. T., et al. 2003. Improved classification of recent HIV-1 infection by employing a two-stage sensitive/less-sensitive test strategy. J. Acquir. Immune Defic. Syndr. 32:94–103 - PubMed
-
- Demmler G. J., Istas A., Easley K. A., Kovacs A. 2000. Results of a quality assurance program for detection of cytomegalovirus infection in the pediatric pulmonary and cardiovascular complications of vertically transmitted human immunodeficiency virus infection study. J. Clin. Microbiol. 38:3942–3945 - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

