Permanent central synaptic disconnection of proprioceptors after nerve injury and regeneration. II. Loss of functional connectivity with motoneurons
- PMID: 21832030
- PMCID: PMC3214087
- DOI: 10.1152/jn.01097.2010
Permanent central synaptic disconnection of proprioceptors after nerve injury and regeneration. II. Loss of functional connectivity with motoneurons
Abstract
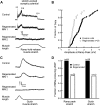
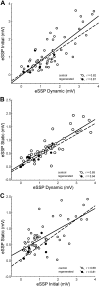
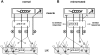
Regeneration of a cut muscle nerve fails to restore the stretch reflex, and the companion paper to this article [Alvarez FJ, Titus-Mitchell HE, Bullinger KL, Kraszpulski M, Nardelli P, Cope TC. J Neurophysiol (August 10, 2011). doi:10.1152/jn.01095.2010] suggests an important central contribution from substantial and persistent disassembly of synapses between regenerated primary afferents and motoneurons. In the present study we tested for physiological correlates of synaptic disruption. Anesthetized adult rats were studied 6 mo or more after a muscle nerve was severed and surgically rejoined. We recorded action potentials (spikes) from individual muscle afferents classified as IA like (*IA) by several criteria and tested for their capacity to produce excitatory postsynaptic potentials (EPSPs) in homonymous motoneurons, using spike-triggered averaging (STA). Nearly every paired recording from a *IA afferent and homonymous motoneuron (93%) produced a STA EPSP in normal rats, but that percentage was only 17% in rats with regenerated nerves. In addition, the number of motoneurons that produced aggregate excitatory stretch synaptic potentials (eSSPs) in response to stretch of the reinnervated muscle was reduced from 100% normally to 60% after nerve regeneration. The decline in functional connectivity was not attributable to synaptic depression, which returned to its normally low level after regeneration. From these findings and those in the companion paper, we put forward a model in which synaptic excitation of motoneurons by muscle stretch is reduced not only by misguided axon regeneration that reconnects afferents to the wrong receptor type but also by retraction of synapses with motoneurons by spindle afferents that successfully reconnect with spindle receptors in the periphery.
Figures



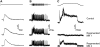

Similar articles
-
Permanent central synaptic disconnection of proprioceptors after nerve injury and regeneration. I. Loss of VGLUT1/IA synapses on motoneurons.J Neurophysiol. 2011 Nov;106(5):2450-70. doi: 10.1152/jn.01095.2010. Epub 2011 Aug 10. J Neurophysiol. 2011. PMID: 21832035 Free PMC article.
-
Central suppression of regenerated proprioceptive afferents.J Neurosci. 2005 May 11;25(19):4733-42. doi: 10.1523/JNEUROSCI.4895-04.2005. J Neurosci. 2005. PMID: 15888649 Free PMC article.
-
Recovery of proprioceptive feedback from nerve crush.J Physiol. 2011 Oct 15;589(Pt 20):4935-47. doi: 10.1113/jphysiol.2011.210518. Epub 2011 Jul 25. J Physiol. 2011. PMID: 21788349 Free PMC article.
-
On the distribution of information from muscle spindles in the spinal cord; how much does it depend on random factors?J Anat. 2015 Aug;227(2):184-93. doi: 10.1111/joa.12331. J Anat. 2015. PMID: 26179024 Free PMC article. Review.
-
Partitioning of monosynaptic Ia excitatory postsynaptic potentials in the motor nucleus of the cat lateral gastrocnemius muscle.J Neurophysiol. 1986 Mar;55(3):569-86. doi: 10.1152/jn.1986.55.3.569. J Neurophysiol. 1986. PMID: 3514815 Review.
Cited by
-
Delayed Onset Muscle Soreness and Critical Neural Microdamage-Derived Neuroinflammation.Biomolecules. 2022 Aug 31;12(9):1207. doi: 10.3390/biom12091207. Biomolecules. 2022. PMID: 36139045 Free PMC article. Review.
-
Neuregulin 1 Drives Morphological and Phenotypical Changes in C2C12 Myotubes: Towards De Novo Formation of Intrafusal Fibres In Vitro.Front Cell Dev Biol. 2022 Jan 11;9:760260. doi: 10.3389/fcell.2021.760260. eCollection 2021. Front Cell Dev Biol. 2022. PMID: 35087826 Free PMC article.
-
Synaptic Plasticity on Motoneurons After Axotomy: A Necessary Change in Paradigm.Front Mol Neurosci. 2020 Apr 30;13:68. doi: 10.3389/fnmol.2020.00068. eCollection 2020. Front Mol Neurosci. 2020. PMID: 32425754 Free PMC article. Review.
-
Patterns of intermuscular inhibitory force feedback across cat hindlimbs suggest a flexible system for regulating whole limb mechanics.J Neurophysiol. 2018 Feb 1;119(2):668-678. doi: 10.1152/jn.00617.2017. Epub 2017 Nov 15. J Neurophysiol. 2018. PMID: 29142095 Free PMC article.
-
Canonical Proprioceptors Are Largely Absent in the Intrinsic Laryngeal Muscles of the Rat Larynx.J Comp Neurol. 2025 Jun;533(6):e70062. doi: 10.1002/cne.70062. J Comp Neurol. 2025. PMID: 40524482 Free PMC article.
References
-
- Abelew TA, Miller MD, Cope TC, Nichols TR. Local loss of proprioception results in disruption of interjoint coordination during locomotion in the cat. J Neurophysiol 84: 2709–2714, 2000 - PubMed
-
- Alvarez FJ, Nardelli P, Bullinger KL, Ukpabi N, Crum JM, Zerda R, Kraszpulski M, Cope TC. VGLUT1 content in central synapses of normal and regenerated Ia afferents (Abstract). 2008 Neuroscience Meeting Planner Washington, DC: Society for Neuroscience, 2008, Program No. 74.1 (online)
-
- Alvarez FJ, Titus-Mitchell HE, Bullinger KL, Kraszpulski M, Nardelli P, Cope TC. Permanent central synaptic disconnection of proprioceptors after nerve injury and regeneration. I. Loss of VGLUT1/IA synapses on motoneurons. J Neurophysiol (August 10, 2011). doi:10.1152/jn.01095.2010 - DOI - PMC - PubMed
-
- Banks RW, Barker D. Neuronal specificities in the reinnervation of muscle spindles. In: Plasticity of Motoneuronal Connections, edited by Wering A. Amsterdam: Elsevier, 1991, p. 241–249
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

