Functional characterization of TNNC1 rare variants identified in dilated cardiomyopathy
- PMID: 21832052
- PMCID: PMC3190822
- DOI: 10.1074/jbc.M111.267211
Functional characterization of TNNC1 rare variants identified in dilated cardiomyopathy
Abstract
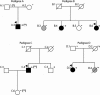
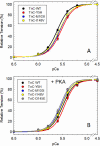
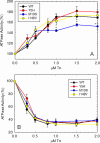
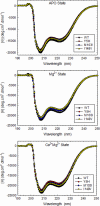
TNNC1, which encodes cardiac troponin C (cTnC), remains elusive as a dilated cardiomyopathy (DCM) gene. Here, we report the clinical, genetic, and functional characterization of four TNNC1 rare variants (Y5H, M103I, D145E, and I148V), all previously reported by us in association with DCM (Hershberger, R. E., Norton, N., Morales, A., Li, D., Siegfried, J. D., and Gonzalez-Quintana, J. (2010) Circ. Cardiovasc. Genet. 3, 155-161); in the previous study, two variants (Y5H and D145E) were identified in subjects who also carried MYH7 and MYBPC3 rare variants, respectively. Functional studies using the recombinant human mutant cTnC proteins reconstituted into porcine papillary skinned fibers showed decreased Ca(2+) sensitivity of force development (Y5H and M103I). Furthermore, the cTnC mutants diminished (Y5H and I148V) or abolished (M103I) the effects of PKA phosphorylation on Ca(2+) sensitivity. Only M103I decreased the troponin activation properties of the actomyosin ATPase when Ca(2+) was present. CD spectroscopic studies of apo (absence of divalent cations)-, Mg(2+)-, and Ca(2+)/Mg(2+)-bound states indicated that all of the cTnC mutants (except I148V in the Ca(2+)/Mg(2+) condition) decreased the α-helical content. These results suggest that each mutation alters the function/ability of the myofilament to bind Ca(2+) as a result of modifications in cTnC structure. One variant (D145E) that was previously reported in association with hypertrophic cardiomyopathy and that produced results in vivo in this study consistent with prior hypertrophic cardiomyopathy functional studies was found associated with the MYBPC3 P910T rare variant, likely contributing to the observed DCM phenotype. We conclude that these rare variants alter the regulation of contraction in some way, and the combined clinical, molecular, genetic, and functional data reinforce the importance of TNNC1 rare variants in the pathogenesis of DCM.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

