The Rho1 GTPase-activating protein CgBem2 is required for survival of azole stress in Candida glabrata
- PMID: 21832071
- PMCID: PMC3190821
- DOI: 10.1074/jbc.M111.264671
The Rho1 GTPase-activating protein CgBem2 is required for survival of azole stress in Candida glabrata
Abstract
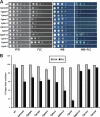
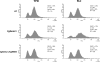
Invasive fungal infections are common clinical complications of neonates, critically ill, and immunocompromised patients worldwide. Candida species are the leading cause of disseminated fungal infections, with Candida albicans being the most prevalent species. Candida glabrata, the second/third most common cause of candidemia, shows reduced susceptibility to a widely used antifungal drug fluconazole. Here, we present findings from a screen of 9134 C. glabrata Tn7 insertion mutants for altered survival profiles in the presence of fluconazole. We have identified two components of RNA polymerase II mediator complex, three players of Rho GTPase-mediated signaling cascade, and two proteins implicated in actin cytoskeleton biogenesis and ergosterol biosynthesis that are required to sustain viability during fluconazole stress. We show that exposure to fluconazole leads to activation of the protein kinase C (PKC)-mediated cell wall integrity pathway in C. glabrata. Our data demonstrate that disruption of a RhoGAP (GTPase activating protein) domain-containing protein, CgBem2, results in bud-emergence defects, azole susceptibility, and constitutive activation of CgRho1-regulated CgPkc1 signaling cascade and cell wall-related phenotypes. The viability loss of Cgbem2Δ mutant upon fluconazole treatment could be partially rescued by the PKC inhibitor staurosporine. Additionally, we present evidence that CgBEM2 is required for the transcriptional activation of genes encoding multidrug efflux pumps in response to fluconazole exposure. Last, we report that Hsp90 inhibitor geldanamycin renders fluconazole a fungicidal drug in C. glabrata.
Figures





Similar articles
-
Loss-of-Function ROX1 Mutations Suppress the Fluconazole Susceptibility of upc2AΔ Mutation in Candida glabrata, Implicating Additional Positive Regulators of Ergosterol Biosynthesis.mSphere. 2021 Dec 22;6(6):e0083021. doi: 10.1128/msphere.00830-21. Epub 2021 Dec 22. mSphere. 2021. PMID: 34935446 Free PMC article.
-
UPC2A is required for high-level azole antifungal resistance in Candida glabrata.Antimicrob Agents Chemother. 2014 Aug;58(8):4543-54. doi: 10.1128/AAC.02217-13. Epub 2014 May 27. Antimicrob Agents Chemother. 2014. PMID: 24867980 Free PMC article.
-
Genetic interaction analysis of Candida glabrata transcription factors CST6 and UPC2A in the regulation of respiration and fluconazole susceptibility.Antimicrob Agents Chemother. 2025 Feb 13;69(2):e0129424. doi: 10.1128/aac.01294-24. Epub 2024 Dec 23. Antimicrob Agents Chemother. 2025. PMID: 39714155 Free PMC article.
-
Candida and candidaemia. Susceptibility and epidemiology.Dan Med J. 2013 Nov;60(11):B4698. Dan Med J. 2013. PMID: 24192246 Review.
-
Resistance in human pathogenic yeasts and filamentous fungi: prevalence, underlying molecular mechanisms and link to the use of antifungals in humans and the environment.Dan Med J. 2016 Oct;63(10):B5288. Dan Med J. 2016. PMID: 27697142 Review.
Cited by
-
Polymorphism of Polymeric Amino Acid Regions in Fungal Proteins and Correlation with Altered Echinocandin and Azole Susceptibility.Antimicrob Agents Chemother. 2018 Nov 26;62(12):e00870-18. doi: 10.1128/AAC.00870-18. Print 2018 Dec. Antimicrob Agents Chemother. 2018. PMID: 30297363 Free PMC article.
-
Loss-of-Function ROX1 Mutations Suppress the Fluconazole Susceptibility of upc2AΔ Mutation in Candida glabrata, Implicating Additional Positive Regulators of Ergosterol Biosynthesis.mSphere. 2021 Dec 22;6(6):e0083021. doi: 10.1128/msphere.00830-21. Epub 2021 Dec 22. mSphere. 2021. PMID: 34935446 Free PMC article.
-
Genome-wide analysis of PTR transporters in Candida species and their functional characterization in Candida auris.Appl Microbiol Biotechnol. 2022 Jun;106(11):4223-4235. doi: 10.1007/s00253-022-11998-9. Epub 2022 Jun 1. Appl Microbiol Biotechnol. 2022. PMID: 35648145
-
Decoding the role of oxidative stress resistance and alternative carbon substrate assimilation in the mature biofilm growth mode of Candida glabrata.BMC Microbiol. 2024 Apr 20;24(1):128. doi: 10.1186/s12866-024-03274-9. BMC Microbiol. 2024. PMID: 38641593 Free PMC article.
-
The effect of biomaterials and antifungals on biofilm formation by Candida species: a review.Eur J Clin Microbiol Infect Dis. 2012 Oct;31(10):2513-27. doi: 10.1007/s10096-012-1634-6. Epub 2012 May 12. Eur J Clin Microbiol Infect Dis. 2012. PMID: 22581304 Review.
References
-
- Grant S. M., Clissold S. P. (1990) Drugs 39, 877–916 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

