Functional characterization of a ficolin-mediated complement pathway in amphioxus
- PMID: 21832079
- PMCID: PMC3196118
- DOI: 10.1074/jbc.M111.245944
Functional characterization of a ficolin-mediated complement pathway in amphioxus
Erratum in
- J Biol Chem. 2012 Feb 3;287(6):4394
Abstract
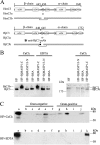
The ficolin-mediated complement pathway plays an important role in vertebrate immunity, but it is not clear whether this pathway exists in invertebrates. Here we identified homologs of ficolin pathway components from the cephalochordate amphioxus and investigated whether they had been co-opted into a functional ficolin pathway. Four of these homologs, ficolin FCN1, serine protease MASP1 and MASP3, and complement component C3, were highly expressed in mucosal tissues and gonads, and were significantly up-regulated following bacterial infection. Recombinant FCN1 could induce hemagglutination, discriminate among sugar components, and specifically recognize and aggregate several bacteria (especially gram-positive strains) without showing bactericidal activity. This suggested that FCN1 is a dedicated pattern-recognition receptor. Recombinant serine protease MASP1/3 formed complexes with recombinant FCN1 and facilitated the activation of native C3 protein in amphioxus humoral fluid, in which C3 acted as an immune effector. We conclude that amphioxus have developed a functional ficolin-complement pathway. Because ficolin pathway components have not been reported in non-chordate species, our findings supported the idea that this pathway may represent a chordate-specific innovation in the evolution of the complement system.
Figures


Similar articles
-
Characterization and comparative analyses of two amphioxus intelectins involved in the innate immune response.Fish Shellfish Immunol. 2013 May;34(5):1139-46. doi: 10.1016/j.fsi.2013.01.017. Epub 2013 Feb 18. Fish Shellfish Immunol. 2013. PMID: 23428515
-
Genome-wide analyses of amphioxus microRNAs reveal an immune regulation via miR-92d targeting C3.J Immunol. 2013 Feb 15;190(4):1491-500. doi: 10.4049/jimmunol.1200801. Epub 2013 Jan 18. J Immunol. 2013. PMID: 23335747
-
Origin of mannose-binding lectin-associated serine protease (MASP)-1 and MASP-3 involved in the lectin complement pathway traced back to the invertebrate, amphioxus.J Immunol. 2003 May 1;170(9):4701-7. doi: 10.4049/jimmunol.170.9.4701. J Immunol. 2003. PMID: 12707349
-
Ficolins: complement-activating lectins involved in innate immunity.J Innate Immun. 2010;2(1):24-32. doi: 10.1159/000228160. Epub 2009 Jul 24. J Innate Immun. 2010. PMID: 20375620 Review.
-
The lectin-complement pathway--its role in innate immunity and evolution.Immunol Rev. 2004 Apr;198:185-202. doi: 10.1111/j.0105-2896.2004.0123.x. Immunol Rev. 2004. PMID: 15199963 Review.
Cited by
-
Identification and characterisation of the immune response properties of Lampetra japonica BLNK.Sci Rep. 2016 Apr 29;6:25308. doi: 10.1038/srep25308. Sci Rep. 2016. PMID: 27126461 Free PMC article.
-
Two apextrin-like proteins mediate extracellular and intracellular bacterial recognition in amphioxus.Proc Natl Acad Sci U S A. 2014 Sep 16;111(37):13469-74. doi: 10.1073/pnas.1405414111. Epub 2014 Sep 3. Proc Natl Acad Sci U S A. 2014. PMID: 25187559 Free PMC article.
-
Integrins meet complement: The evolutionary tip of an iceberg orchestrating metabolism and immunity.Br J Pharmacol. 2021 Jul;178(14):2754-2770. doi: 10.1111/bph.15168. Epub 2020 Jul 19. Br J Pharmacol. 2021. PMID: 32562277 Free PMC article. Review.
-
Identification and characterization of the lamprey high-mobility group box 1 gene.PLoS One. 2012;7(4):e35755. doi: 10.1371/journal.pone.0035755. Epub 2012 Apr 26. PLoS One. 2012. PMID: 22563397 Free PMC article.
-
Hepatic cecum: a key integrator of immunity in amphioxus.Mar Life Sci Technol. 2021 Jan 2;3(3):279-292. doi: 10.1007/s42995-020-00080-w. eCollection 2021 Aug. Mar Life Sci Technol. 2021. PMID: 37073295 Free PMC article. Review.
References
-
- Köhl J. (2006) Adv. Exp. Med. Biol. 586, 71–94 - PubMed
-
- Sunyer J. O., Lambris J. D. (1998) Immunol. Rev. 166, 39–57 - PubMed
-
- Fujita T., Matsushita M., Endo Y. (2004) Immunol. Rev. 198, 185–202 - PubMed
-
- Arlaud G. J., Gaboriaud C., Thielens N. M., Rossi V. (2002) Biochem. Soc. Trans 30, 1001–1006 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

