Phospholamban binds with differential affinity to calcium pump conformers
- PMID: 21832088
- PMCID: PMC3186385
- DOI: 10.1074/jbc.M111.266759
Phospholamban binds with differential affinity to calcium pump conformers
Abstract
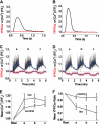
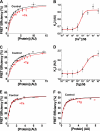
To investigate the mechanism of regulation of sarco-endoplasmic reticulum Ca(2+)-ATPase (SERCA) by phospholamban (PLB), we expressed Cerulean-SERCA and yellow fluorescent protein (YFP)-PLB in adult rabbit ventricular myocytes using adenovirus vectors. SERCA and PLB were localized in the sarcoplasmic reticulum and were mobile over multiple sarcomeres on a timescale of tens of seconds. We also observed robust fluorescence resonance energy transfer (FRET) from Cerulean-SERCA to YFP-PLB. Electrical pacing of cardiac myocytes elicited cytoplasmic Ca(2+) elevations, but these increases in Ca(2+) produced only modest changes in SERCA-PLB FRET. The data suggest that the regulatory complex is not disrupted by elevations of cytosolic calcium during cardiac contraction (systole). This conclusion was also supported by parallel experiments in heterologous cells, which showed that FRET was reduced but not abolished by calcium. Thapsigargin also elicited a small decrease in PLB-SERCA binding affinity. We propose that PLB is not displaced from SERCA by high calcium during systole, and relief of functional inhibition does not require dissociation of the regulatory complex. The observed modest reduction in the affinity of the PLB-SERCA complex with Ca(2+) or thapsigargin suggests that the binding interface is altered by SERCA conformational changes. The results are consistent with multiple modes of PLB binding or alternative binding sites.
Figures



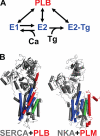
Similar articles
-
Phospholamban oligomerization, quaternary structure, and sarco(endo)plasmic reticulum calcium ATPase binding measured by fluorescence resonance energy transfer in living cells.J Biol Chem. 2008 May 2;283(18):12202-11. doi: 10.1074/jbc.M707590200. Epub 2008 Feb 19. J Biol Chem. 2008. PMID: 18287099 Free PMC article.
-
Phospholamban C-terminal residues are critical determinants of the structure and function of the calcium ATPase regulatory complex.J Biol Chem. 2014 Sep 12;289(37):25855-66. doi: 10.1074/jbc.M114.562579. Epub 2014 Jul 29. J Biol Chem. 2014. PMID: 25074938 Free PMC article.
-
Direct detection of phospholamban and sarcoplasmic reticulum Ca-ATPase interaction in membranes using fluorescence resonance energy transfer.Biochemistry. 2004 Jul 13;43(27):8754-65. doi: 10.1021/bi049732k. Biochemistry. 2004. PMID: 15236584
-
Phospholamban and sarcolipin: Are they functionally redundant or distinct regulators of the Sarco(Endo)Plasmic Reticulum Calcium ATPase?J Mol Cell Cardiol. 2016 Feb;91:81-91. doi: 10.1016/j.yjmcc.2015.12.030. Epub 2015 Dec 29. J Mol Cell Cardiol. 2016. PMID: 26743715 Free PMC article. Review.
-
SR Ca(2+)-ATPase/phospholamban in cardiomyocyte function.J Card Fail. 1996 Dec;2(4 Suppl):S77-85. doi: 10.1016/s1071-9164(96)80062-5. J Card Fail. 1996. PMID: 8951564 Review.
Cited by
-
The Phospholamban Pentamer Alters Function of the Sarcoplasmic Reticulum Calcium Pump SERCA.Biophys J. 2019 Feb 19;116(4):633-647. doi: 10.1016/j.bpj.2019.01.013. Epub 2019 Jan 22. Biophys J. 2019. PMID: 30712785 Free PMC article.
-
Atomic-level mechanisms for phospholamban regulation of the calcium pump.Biophys J. 2015 Apr 7;108(7):1697-1708. doi: 10.1016/j.bpj.2015.03.004. Biophys J. 2015. PMID: 25863061 Free PMC article.
-
The structural basis for phospholamban inhibition of the calcium pump in sarcoplasmic reticulum.J Biol Chem. 2013 Oct 18;288(42):30181-30191. doi: 10.1074/jbc.M113.501585. Epub 2013 Aug 31. J Biol Chem. 2013. PMID: 23996003 Free PMC article.
-
A separate pool of cardiac phospholemman that does not regulate or associate with the sodium pump: multimers of phospholemman in ventricular muscle.J Biol Chem. 2013 May 10;288(19):13808-20. doi: 10.1074/jbc.M113.460956. Epub 2013 Mar 26. J Biol Chem. 2013. PMID: 23532852 Free PMC article.
-
Structural basis for allosteric control of the SERCA-Phospholamban membrane complex by Ca2+ and phosphorylation.Elife. 2021 May 12;10:e66226. doi: 10.7554/eLife.66226. Elife. 2021. PMID: 33978571 Free PMC article.
References
-
- Hasenfuss G., Reinecke H., Studer R., Meyer M., Pieske B., Holtz J., Holubarsch C., Posival H., Just H., Drexler H. (1994) Circ. Res. 75, 434–442 - PubMed
-
- Schmitt J. P., Kamisago M., Asahi M., Li G. H., Ahmad F., Mende U., Kranias E. G., MacLennan D. H., Seidman J. G., Seidman C. E. (2003) Science 299, 1410–1413 - PubMed
-
- MacLennan D. H., Kranias E. G. (2003) Nat. Rev. Mol. Cell Biol. 4, 566–577 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

