Metformin and/or clomiphene do not adversely affect liver or renal function in women with polycystic ovary syndrome
- PMID: 21832111
- PMCID: PMC3200244
- DOI: 10.1210/jc.2011-1093
Metformin and/or clomiphene do not adversely affect liver or renal function in women with polycystic ovary syndrome
Abstract
Context: Nonalcoholic fatty liver disease is common to insulin-resistant states such as polycystic ovary syndrome (PCOS). Metformin (MET) is often used to treat PCOS but information is limited as to its effects on liver function.
Objective: We sought to determine the effects of MET on serum hepatic parameters in PCOS patients.
Design: This was a secondary analysis of a randomized, doubled-blind trial from 2002-2004.
Setting: This multi-center clinical trial was conducted in academic centers.
Patients: Six hundred twenty-six infertile women with PCOS with serum liver function parameters less than twice the upper limit of normal were included.
Interventions: Clomiphene citrate (n = 209), MET (n = 208), or combined (n = 209) were given for up to 6 months.
Main outcome measure: The percent change from baseline in renal and liver function between- and within-treatment arms was assessed.
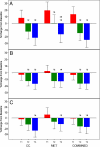
Results: Renal function improved in all treatment arms with significant decreases in serum blood urea nitrogen levels (range, -14.7 to -21.3%) as well as creatinine (-4.2 to -6.9%). There were similar decreases in liver transaminase levels in the clomiphene citrate and combined arms (-10% in bilirubin, -9 to -11% in transaminases) without significant changes in the MET arm. When categorizing baseline bilirubin, aspartate aminotransferase, and alanine aminotransferase into tertiles, there were significant within-treatment arm differences between the tertiles with the highest tertile having the largest decrease from baseline regardless of treatment arm.
Conclusion: Women with PCOS can safely use metformin and clomiphene even in the setting of mildly abnormal liver function parameters, and both result in improved renal function.
Trial registration: ClinicalTrials.gov NCT00068861.
Figures
References
-
- Legro RS, Myers ER, Barnhart HX, Carson SA, Diamond MP, Carr BR, Schlaff WD, Coutifaris C, McGovern PG, Cataldo NA, Steinkampf MP, Nestler JE, Gosman G, Guidice LC, Leppert PC. 2006. The pregnancy in polycystic ovary syndrome study: baseline characteristics of the randomized cohort including racial effects. Fertil Steril 86:914–933 - PubMed
-
- Babich MM, Pike I, Shiffman ML. 1998. Metformin-induced acute hepatitis. Am J Med 104:490–492 - PubMed
-
- Deutsch M, Kountouras D, Dourakis SP. 2004. Metformin hepatotoxicity. Ann Intern Med 140:W25. - PubMed
-
- Kutoh E. 2005. Possible metformin-induced hepatotoxicity. Am J Geriatr Pharmacother 3:270–273 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- U10 HD038988/HD/NICHD NIH HHS/United States
- U10 HD039005/HD/NICHD NIH HHS/United States
- U10 HD27049/HD/NICHD NIH HHS/United States
- U10 HD055944/HD/NICHD NIH HHS/United States
- U10 HD033172/HD/NICHD NIH HHS/United States
- U10 HD027049/HD/NICHD NIH HHS/United States
- U10 HD27011/HD/NICHD NIH HHS/United States
- U10 HD38988/HD/NICHD NIH HHS/United States
- U10 HD027011/HD/NICHD NIH HHS/United States
- M01 RR010732/RR/NCRR NIH HHS/United States
- MO1RR00056/RR/NCRR NIH HHS/United States
- H10 HD055944/HD/NICHD NIH HHS/United States
- U10 HD33172/HD/NICHD NIH HHS/United States
- M01 RR000056/RR/NCRR NIH HHS/United States
- U10 HD055936/HD/NICHD NIH HHS/United States
- C06 RR016499/RR/NCRR NIH HHS/United States
- U10 HD055925/HD/NICHD NIH HHS/United States
- MO1RR10732/RR/NCRR NIH HHS/United States
- U10 HD38998/HD/NICHD NIH HHS/United States
- U10 HD038998/HD/NICHD NIH HHS/United States
- U10 HD39005/HD/NICHD NIH HHS/United States
- U10 HD038992/HD/NICHD NIH HHS/United States
- U10 HD38992/HD/NICHD NIH HHS/United States
- U10 HD38999/HD/NICHD NIH HHS/United States
- U10 HD038999/HD/NICHD NIH HHS/United States
- U10HD055925/HD/NICHD NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

