TRAIL/DR5 plays a critical role in NK cell-mediated negative regulation of dendritic cell cross-priming of T cells
- PMID: 21832159
- PMCID: PMC3169733
- DOI: 10.4049/jimmunol.1003879
TRAIL/DR5 plays a critical role in NK cell-mediated negative regulation of dendritic cell cross-priming of T cells
Abstract
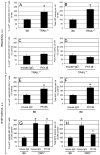
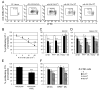
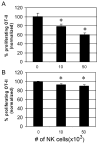
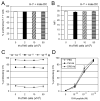
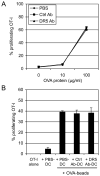
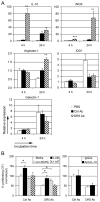
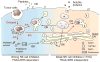
Dendritic cells (DCs) are critical in initiating immune responses by cross-priming of tumor Ags to T cells. Previous results showed that NK cells inhibited DC-mediated cross-presentation of tumor Ags both in vivo and in vitro. In this study, enhanced Ag presentation was observed in draining lymph nodes in TRAIL(-/-) and DR5(-/-) mice compared with that of wild-type mice. NK cells inhibit DC cross-priming of tumor Ags in vitro, but not direct presentation of endogenous Ags. NK cells lacking TRAIL, but not perforin, were not able to inhibit DC cross-priming of tumor Ags. DCs that lack expression of TRAIL receptor DR5 were less susceptible to NK cell-mediated inhibition of cross-priming, and cross-linking of DR5 receptor led to reduced generation of MHC class I-Ag peptide complexes, followed by attenuated cross-priming of CD8(+) T cells. In addition, key molecules involved in the TRAIL/DR5 pathway during DC/NK cell interactions were determined. In summary, these data indicate a novel alternative pathway for DC/NK cell interactions in antitumor immunity and may reflect homeostasis of both DCs and NK cells for regulation of CD8(+) T cell function in physiological conditions.
Conflict of interest statement
The authors declare no financial or commercial conflict of interest.
Figures

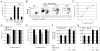
References
-
- Lanier LL. NK cell recognition. Annu Rev Immunol. 2005;23:225–274. - PubMed
-
- Degli-Esposti MA, Smyth MJ. Close encounters of different kinds: dendritic cells and NK cells take centre stage. Nat Rev Immunol. 2005;5:112–124. - PubMed
-
- Walzer T, Dalod M, Robbins SH, Zitvogel L, Vivier E. Natural-killer cells and dendritic cells: “l’union fait la force”. Blood. 2005;106:2252–2258. - PubMed
-
- Tacken PJ, I, de Vries J, Torensma R, Figdor CG. Dendritic-cell immunotherapy: from ex vivo loading to in vivo targeting. Nat Rev Immunol. 2007;7:790–802. - PubMed
-
- Shen L, Rock KL. Priming of T cells by exogenous antigen cross-presented on MHC class I molecules. Curr Opin Immunol. 2006;18:85–91. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

