Inducible nitric oxide synthase (iNOS) in muscle wasting syndrome, sarcopenia, and cachexia
- PMID: 21832306
- PMCID: PMC3184974
- DOI: 10.18632/aging.100358
Inducible nitric oxide synthase (iNOS) in muscle wasting syndrome, sarcopenia, and cachexia
Abstract
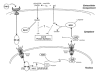
Muscle atrophy-also known as muscle wasting-is a debilitating syndrome that slowly develops with age (sarcopenia) or rapidly appears at the late stages of deadly diseases such as cancer, AIDS, and sepsis (cachexia). Despite the prevalence and the drastic detrimental effects of these two syndromes, there are currently no widely used, effective treatment options for those suffering from muscle wasting. In an attempt to identify potential therapeutic targets, the molecular mechanisms of sarcopenia and cachexia have begun to be elucidated. Growing evidence suggests that inflammatory cytokines may play an important role in the pathology of both syndromes. As one of the key cytokines involved in both sarcopenic and cachectic muscle wasting, tumor necrosis factor α (TNFα) and its downstream effectors provide an enticing target for pharmacological intervention. However, to date, no drugs targeting the TNFα signaling pathway have been successful as a remedial option for the treatment of muscle wasting. Thus, there is a need to identify new effectors in this important pathway that might prove to be more efficacious targets. Inducible nitric oxide synthase (iNOS) has recently been shown to be an important mediator of TNFα-induced cachectic muscle loss, and studies suggest that it may also play a role in sarcopenia. In addition, investigations into the mechanism of iNOS-mediated muscle loss have begun to reveal potential therapeutic strategies. In this review, we will highlight the potential for targeting the iNOS/NO pathway in the treatment of muscle loss and discuss its functional relevance in sarcopenia and cachexia.
Figures
Similar articles
-
eIF4A inhibition prevents the onset of cytokine-induced muscle wasting by blocking the STAT3 and iNOS pathways.Sci Rep. 2018 May 30;8(1):8414. doi: 10.1038/s41598-018-26625-9. Sci Rep. 2018. PMID: 29849089 Free PMC article.
-
Disrupted NOS2 metabolism drives myoblast response to wasting-associated cytokines.Exp Cell Res. 2021 Oct 1;407(1):112779. doi: 10.1016/j.yexcr.2021.112779. Epub 2021 Aug 21. Exp Cell Res. 2021. PMID: 34428455 Free PMC article.
-
Molecular mechanisms involved in muscle wasting in cancer and ageing: cachexia versus sarcopenia.Int J Biochem Cell Biol. 2005 May;37(5):1084-104. doi: 10.1016/j.biocel.2004.10.003. Epub 2004 Dec 30. Int J Biochem Cell Biol. 2005. PMID: 15743680 Review.
-
Muscle wasting in cancer and ageing: cachexia versus sarcopenia.Adv Gerontol. 2006;18:39-54. Adv Gerontol. 2006. PMID: 16676797 Review.
-
Pharmacological or genetic inhibition of iNOS prevents cachexia-mediated muscle wasting and its associated metabolism defects.EMBO Mol Med. 2021 Jul 7;13(7):e13591. doi: 10.15252/emmm.202013591. Epub 2021 Jun 7. EMBO Mol Med. 2021. PMID: 34096686 Free PMC article.
Cited by
-
Pancreatic cancer cachexia: three dimensions of a complex syndrome.Br J Cancer. 2021 May;124(10):1623-1636. doi: 10.1038/s41416-021-01301-4. Epub 2021 Mar 19. Br J Cancer. 2021. PMID: 33742145 Free PMC article. Review.
-
Surgery-Related Muscle Loss and Its Association with Postoperative Complications After Major Hepatectomy with Extrahepatic Bile Duct Resection.World J Surg. 2017 Feb;41(2):498-507. doi: 10.1007/s00268-016-3732-6. World J Surg. 2017. PMID: 27718001
-
HuR counteracts miR-330 to promote STAT3 translation during inflammation-induced muscle wasting.Proc Natl Acad Sci U S A. 2019 Aug 27;116(35):17261-17270. doi: 10.1073/pnas.1905172116. Epub 2019 Aug 12. Proc Natl Acad Sci U S A. 2019. PMID: 31405989 Free PMC article.
-
Sarcopenia: investigation of metabolic changes and its associated mechanisms.Skelet Muscle. 2023 Jan 19;13(1):2. doi: 10.1186/s13395-022-00312-w. Skelet Muscle. 2023. PMID: 36658632 Free PMC article.
-
Molecular regulation of skeletal muscle mass and the contribution of nitric oxide: A review.FASEB Bioadv. 2019 Apr 11;1(6):364-374. doi: 10.1096/fba.2018-00080. eCollection 2019 Jun. FASEB Bioadv. 2019. PMID: 32123839 Free PMC article.
References
-
- Rolland Y, Van Kan GA, Gillette-Guyonnet S, Vellas B. Cachexia versus sarcopenia. Curr Opin Clin Nutr Metab Care. 2011;14:15–21. - PubMed
-
- Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, Martin FC, Michel JP, Rolland Y, Schneider SM, Topinkova E, Vandewoude M, Zamboni M. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39:412–423. - PMC - PubMed
-
- Fielding RA, Vellas B, Evans WJ, Bhasin S, Morley JE, Newman AB, Abellan van Kan G, Andrieu S, Bauer J, Breuille D, Cederholm T, Chandler J, De Meynard C, Donini L, Harris T, Kannt A, et al. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International working group on sarcopenia. J Am Med Dir Assoc. 2011;12:249–256. - PMC - PubMed
-
- Argiles JM, Busquets S, Felipe A, Lopez-Soriano FJ. Molecular mechanisms involved in muscle wasting in cancer and ageing: cachexia versus sarcopenia. Int J Biochem Cell Biol. 2005;37:1084–1104. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

