Two independent networks of interstitial cells of cajal work cooperatively with the enteric nervous system to create colonic motor patterns
- PMID: 21833164
- PMCID: PMC3153851
- DOI: 10.3389/fnins.2011.00093
Two independent networks of interstitial cells of cajal work cooperatively with the enteric nervous system to create colonic motor patterns
Abstract
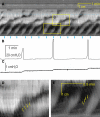
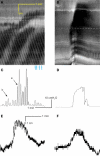
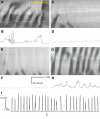
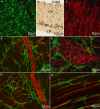
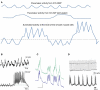
Normal motility of the colon is critical for quality of life and efforts to normalize abnormal colon function have had limited success. A better understanding of control systems of colonic motility is therefore essential. We report here a hypothesis with supporting experimental data to explain the origin of rhythmic propulsive colonic motor activity induced by general distention. The theory holds that both networks of interstitial cells of Cajal (ICC), those associated with the submuscular plexus (ICC-SMP) and those associated with the myenteric plexus (ICC-MP), orchestrate propagating contractions as pacemaker cells in concert with the enteric nervous system (ENS). ICC-SMP generate an omnipresent slow wave activity that causes propagating but non-propulsive contractions ("rhythmic propagating ripples") enhancing absorption. The ICC-MP generate stimulus-dependent cyclic depolarizations propagating anally and directing propulsive activity ("rhythmic propulsive motor complexes"). The ENS is not essential for both rhythmic motor patterns since distention and pharmacological means can produce the motor patterns after blocking neural activity, but it supplies the primary stimulus in vivo. Supporting data come from studies on segments of the rat colon, simultaneously measuring motility through spatiotemporal mapping of video recordings, intraluminal pressure, and outflow measurements.
Keywords: ENS; ICC; colon; colonic motility; peristalsis.
Figures
References
-
- Barajas-Lopez C., Huizinga J. D. (1989). Different mechanisms of contraction generation in circular muscle of canine colon. Am. J. Physiol. 256, G570–G580 - PubMed
-
- Bauer A. J., Publicover N. G., Sanders K. M. (1985). Origin and spread of slow waves in canine gastric antral circular muscle. Am. J. Physiol. 249, G800–G806 - PubMed
LinkOut - more resources
Full Text Sources

