Identification of Novel CYP2D7-2D6 Hybrids: Non-Functional and Functional Variants
- PMID: 21833166
- PMCID: PMC3153001
- DOI: 10.3389/fphar.2010.00121
Identification of Novel CYP2D7-2D6 Hybrids: Non-Functional and Functional Variants
Abstract
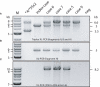
Polymorphic expression of CYP2D6 contributes to the wide range of activity observed for this clinically important drug metabolizing enzyme. In this report we describe novel CYP2D7/2D6 hybrid genes encoding non-functional and functional CYP2D6 protein and a CYP2D7 variant that mimics a CYP2D7/2D6 hybrid gene. Five-kilobyte-long PCR products encompassing the novel genes were entirely sequenced. A quantitative assay probing in different gene regions was employed to determine CYP2D6 and 2D7 copy number variations and the relative position of the hybrid genes within the locus was assessed by long-range PCR. In addition to the previously known CYP2D6*13 and *66 hybrids, we describe three novel non-functional CYP2D7-2D6 hybrids with gene switching in exon 2 (CYP2D6*79), intron 2 (CYP2D6*80), and intron 5 (CYP2D6*67). A CYP2D7-specific T-ins in exon 1 causes a detrimental frame shift. One subject revealed a CYP2D7 conversion in the 5'-flanking region of a CYP2D6*35 allele, was otherwise unaffected (designated CYP2D6*35B). Finally, three DNAs revealed a CYP2D7 gene with a CYP2D6-like region downstream of exon 9 (designated CYP2D7[REP6]). Quantitative copy number determination, sequence analyses, and long-range PCR mapping were in agreement and excluded the presence of additional gene units. Undetected hybrid genes may cause over-estimation of CYP2D6 activity (CYP2D6*1/*1 vs *1/hybrid, etc), but may also cause results that may interfere with the genotype determination. Detection of hybrid events, "single" and tandem, will contribute to more accurate phenotype prediction from genotype data.
Keywords: CYP2D6; CYP2D6 poor metabolizer; CYP2D6*35B; CYP2D6*67; CYP2D6*79; CYP2D6*80; hybrid genes.
Figures




References
-
- Daly A. K., Fairbrother K. S., Andreassen O. A., London S. J., Idle J. R., Steen V. M. (1996). Characterization and PCR-based detection of two different hybrid CYP2D7P/CYP2D6 alleles associated with the poor metabolizer phenotype. Pharmacogenetics 6, 319–328 10.1097/00008571-199608000-00005 - DOI - PubMed
-
- de Leon J., Susce M. T., Johnson M., Hardin M., Maw L., Shao A., Allen A. C., Chiafari F. A., Hillman G., Nikoloff D. M. (2009). DNA microarray technology in the clinical environment: the AmpliChip CYP450 test for CYP2D6 and CYP2C19 genotyping. CNS Spectrums 14, 19–34 - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

