Molecular Pharmacology of Kidney and Inner Ear CLC-K Chloride Channels
- PMID: 21833170
- PMCID: PMC3153005
- DOI: 10.3389/fphar.2010.00130
Molecular Pharmacology of Kidney and Inner Ear CLC-K Chloride Channels
Abstract
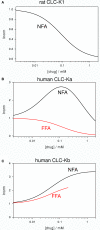
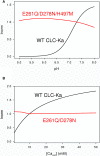
CLC-K channels belong to the CLC gene family, which comprises both Cl(-) channels and Cl(-)/H(+) antiporters. They form homodimers which additionally co-assemble with the small protein barttin. In the kidney, they are involved in NaCl reabsorption; in the inner ear they are important for endolymph production. Mutations in CLC-Kb lead to renal salt loss (Bartter's syndrome); mutations in barttin lead additionally to deafness. CLC-K channels are interesting potential drug targets. CLC-K channel blockers have potential as alternative diuretics, whereas CLC-K activators could be used for the treatment of patients with Bartter's syndrome. Several small organic acids inhibit CLC-K channels from the outside by binding to a site in the external vestibule of the ion conducting pore. Benzofuran derivatives with affinities better than 10 μM have been discovered. Niflumic acid (NFA) exhibits a complex interaction with CLC-K channels. Below ∼1 mM, NFA activates CLC-Ka, whereas at higher concentrations NFA inhibits channel activity. The co-planarity of the rings of the NFA molecule is essential for its activating action. Mutagenesis has led to the identification of potential regions of the channel that interact with NFA. CLC-K channels are also modulated by pH and [Ca(2+)](ext). The inhibition at low pH has been shown to be mediated by a His-residue at the beginning of helix Q, the penultimate transmembrane helix. Two acidic residues from opposite subunits form two symmetrically related intersubunit Ca(2+) binding sites, whose occupation increases channel activity. The relatively high affinity CLC-K blockers may already serve as leads for the development of useful drugs. On the other hand, the CLC-K potentiator NFA has a quite low affinity, and, being a non-steroidal anti-inflammatory drug, can be expected to exert significant side effects. More specific and more potent activators will be needed and it will be important to understand the molecular mechanisms that underlie NFA activation.
Keywords: CLC; calcium; chloride channel; chloride transport; diuretic; fenamates; inner ear; kidney.
Figures

Similar articles
-
Mechanism of interaction of niflumic acid with heterologously expressed kidney CLC-K chloride channels.J Membr Biol. 2007 Apr;216(2-3):73-82. doi: 10.1007/s00232-007-9034-z. Epub 2007 Jul 21. J Membr Biol. 2007. PMID: 17659402
-
Identification of sites responsible for the potentiating effect of niflumic acid on ClC-Ka kidney chloride channels.Br J Pharmacol. 2010 Aug;160(7):1652-61. doi: 10.1111/j.1476-5381.2010.00822.x. Br J Pharmacol. 2010. PMID: 20649569 Free PMC article.
-
Activation and inhibition of kidney CLC-K chloride channels by fenamates.Mol Pharmacol. 2006 Jan;69(1):165-73. doi: 10.1124/mol.105.017384. Epub 2005 Oct 21. Mol Pharmacol. 2006. PMID: 16244177
-
Small Molecules Targeting Kidney ClC-K Chloride Channels: Applications in Rare Tubulopathies and Common Cardiovascular Diseases.Biomolecules. 2023 Apr 21;13(4):710. doi: 10.3390/biom13040710. Biomolecules. 2023. PMID: 37189456 Free PMC article. Review.
-
How Bartter's and Gitelman's syndromes, and Dent's disease have provided important insights into the function of three renal chloride channels: ClC-Ka/b and ClC-5.Nephron Physiol. 2006;103(1):p7-13. doi: 10.1159/000090218. Epub 2005 Dec 12. Nephron Physiol. 2006. PMID: 16352917 Review.
Cited by
-
Cochlear protein biomarkers as potential sites for targeted inner ear drug delivery.Drug Deliv Transl Res. 2020 Apr;10(2):368-379. doi: 10.1007/s13346-019-00692-5. Drug Deliv Transl Res. 2020. PMID: 31741303 Free PMC article. Review.
-
Positive modulation of the TMEM16B mediated currents by TRPV4 antagonist.Biochem Biophys Rep. 2021 Dec 2;28:101180. doi: 10.1016/j.bbrep.2021.101180. eCollection 2021 Dec. Biochem Biophys Rep. 2021. PMID: 34917777 Free PMC article.
-
In silico model of the human ClC-Kb chloride channel: pore mapping, biostructural pathology and drug screening.Sci Rep. 2017 Aug 3;7(1):7249. doi: 10.1038/s41598-017-07794-5. Sci Rep. 2017. PMID: 28775266 Free PMC article.
-
Alkaline pH block of CLC-K kidney chloride channels mediated by a pore lysine residue.Biophys J. 2013 Jul 2;105(1):80-90. doi: 10.1016/j.bpj.2013.05.044. Biophys J. 2013. PMID: 23823226 Free PMC article.
-
I-J loop involvement in the pharmacological profile of CLC-K channels expressed in Xenopus oocytes.Biochim Biophys Acta. 2014 Nov;1838(11):2745-56. doi: 10.1016/j.bbamem.2014.07.021. Epub 2014 Jul 26. Biochim Biophys Acta. 2014. PMID: 25073071 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

