Neural markers of opposite-sex bias in face processing
- PMID: 21833232
- PMCID: PMC3153781
- DOI: 10.3389/fpsyg.2010.00169
Neural markers of opposite-sex bias in face processing
Abstract
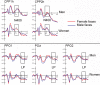
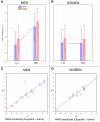
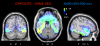
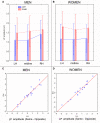
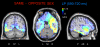
Some behavioral and neuroimaging studies suggest that adults prefer to view attractive faces of the opposite sex more than attractive faces of the same sex. However, unlike the other-race face effect (Caldara et al., 2004), little is known regarding the existence of an opposite-/same-sex bias in face processing. In this study, the faces of 130 attractive male and female adults were foveally presented to 40 heterosexual university students (20 men and 20 women) who were engaged in a secondary perceptual task (landscape detection). The automatic processing of face gender was investigated by recording ERPs from 128 scalp sites. Neural markers of opposite- vs. same-sex bias in face processing included larger and earlier centro-parietal N400s in response to faces of the opposite sex and a larger late positivity (LP) to same-sex faces. Analysis of intra-cortical neural generators (swLORETA) showed that facial processing-related (FG, BA37, BA20/21) and emotion-related brain areas (the right parahippocampal gyrus, BA35; uncus, BA36/38; and the cingulate gyrus, BA24) had higher activations in response to opposite- than same-sex faces. The results of this analysis, along with data obtained from ERP recordings, support the hypothesis that both genders process opposite-sex faces differently than same-sex faces. The data also suggest a hemispheric asymmetry in the processing of opposite-/same-sex faces, with the right hemisphere involved in processing same-sex faces and the left hemisphere involved in processing faces of the opposite sex. The data support previous literature suggesting a right lateralization for the representation of self-image and body awareness.
Keywords: ERPs; body awareness; face coding; hemispheric asymmetry; self-representation; sex differences; social cognition; visual perception.
Figures




References
-
- Byatt G., Rhodes G. (2004). Identification of own-race and other-race faces: implications for the representation of race in face space. Psychon. Bull. Rev. 11, 735–741 - PubMed
LinkOut - more resources
Full Text Sources

