Attachment of HeLa cells during early G1 phase
- PMID: 21833479
- PMCID: PMC3174367
- DOI: 10.1007/s00418-011-0852-9
Attachment of HeLa cells during early G1 phase
Abstract
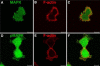
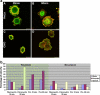
Both growth factor directed and integrin dependent signal transduction were shown to take place directly after completion of mitosis. The local activation of these signal transduction cascades was investigated in early G1 cells. Interestingly, various key signal transduction proteins were found in blebs at the cell membrane within 30 min after mitosis. These membrane blebs appeared in round, mitotic-like cells and disappeared rapidly during spreading of the cells in G1 phase. In addition to tyrosine-phosphorylated proteins, the blebs contained also phosphorylated FAK and phosphorylated MAP kinase. The formation of membrane blebs in round, mitotic cells before cell spreading is not specific for mitotic cells, because similar features were observed in trypsinized cells. Just before cell spreading also these cells exhibited membrane blebs containing active signal transduction proteins. Inhibition of signal transduction did not affect membrane bleb formation, suggesting that the membrane blebs were formed independent of signal transduction.
Figures







References
-
- Badley RA, Woods A, Carruthers L, Rees DA. Cytoskeleton changes in fibroblast adhesion and detachment. J Cell Sci. 1980;43:379–390. - PubMed
-
- Boonstra J, Moes MJ. Signal transduction and actin in the regulation of G1-phase progression. Crit Rev Eukaryot Gene Expr. 2005;15:255–276. - PubMed
-
- Bottazzi MA, Buzzai M, Zhu X, Desdouets C, Brechot C, Assoian RK. Distinct effects of mitogens and the actin cytoskeleton on CREB and pocket protein phosphorylation control the extent and timing of cyclin A promoter activity. Mol Cell Biol. 2001;21:7607–7616. doi: 10.1128/MCB.21.22.7607-7616.2001. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

