Molecular mechanisms of muscarinic acetylcholine receptor-stimulated increase in cytosolic free Ca(2+) concentration and ERK1/2 activation in the MIN6 pancreatic β-cell line
- PMID: 21833779
- PMCID: PMC3407357
- DOI: 10.1007/s00592-011-0314-9
Molecular mechanisms of muscarinic acetylcholine receptor-stimulated increase in cytosolic free Ca(2+) concentration and ERK1/2 activation in the MIN6 pancreatic β-cell line
Abstract
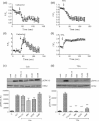
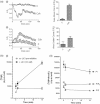
Muscarinic acetylcholine receptor (mAChR) activation of pancreatic β-cells elevates intracellular Ca(2+) and potentiates glucose-stimulated insulin secretion. In addition, it activates a number of signaling molecules, including ERK1/2, whose activation has been shown to play an important role in regulating pancreatic β-cell function and mass. The aim of this work was to determine how mAChR activation elevates intracellular Ca(2+) concentration ([Ca(2+)]( i )) and activates ERK1/2 in the pancreatic β-cell line MIN6. We demonstrate that agonist-stimulated ERK1/2 activation is dependent on the activation of phospholipase C and an elevation in [Ca(2+)]( i ), but is independent of the activation of diacylglycerol-dependent protein kinase C isoenzymes. Using a pharmacological approach, we provide evidence that agonist-induced increases in [Ca(2+)]( i ) and ERK activity require (1) IP(3) receptor-mediated mobilization of Ca(2+) from the endoplasmic reticulum, (2) influx of extracellular Ca(2+) through store-operated channels, (3) closure of K(ATP) channels, and (4) Ca(2+) entry via L-type voltage-operated Ca(2+) channels. Moreover, this Ca(2+)-dependent activation of ERK is mediated via both Ras-dependent and Ras-independent mechanisms. In summary, this study provides important insights into the multifactorial signaling mechanisms linking mAChR activation to increases in [Ca(2+)]( i ) and ERK activity.
Figures





References
-
- Gautam D, Han SJ, Hamdan FF, Jeon J, Li B, Li JH, Cui Y, Mears D, Lu H, Deng C, Heard T, Wess J. A critical role for beta cell M3 muscarinic acetylcholine receptors in regulating insulin release and blood glucose homeostasis in vivo. Cell Metab. 2006;3(6):449–461. doi: 10.1016/j.cmet.2006.04.009. - DOI - PubMed
-
- Kanat M, Norton L, Winnier D, Jenkinson C, Defronzo RA, Abdul-Ghani MA Impaired early- but not late-phase insulin secretion in subjects with impaired fasting glucose. Acta Diabetol. doi:10.1007/s00592-011-0285-x - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

