Chemistry and biology of rocaglamides (= flavaglines) and related derivatives from aglaia species (meliaceae)
- PMID: 21833837
- PMCID: PMC4157394
- DOI: 10.1007/978-3-7091-0748-5_1
Chemistry and biology of rocaglamides (= flavaglines) and related derivatives from aglaia species (meliaceae)
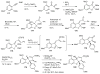
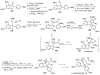
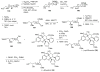
Figures







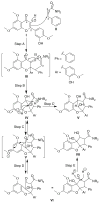
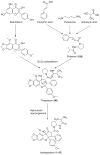






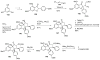
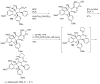


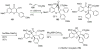





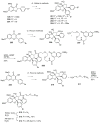
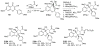


References
-
- Newman DJ, Cragg GM, Snader KM. The Influence of Natural Products upon Drug Discovery. Nat Prod Rep. 2000;17:215. - PubMed
-
- Butler MS. Natural Products to Drugs: Natural Product-Derived Compounds in Clinical Trials. Nat Prod Rep. 2008;25:475. - PubMed
-
- Altmann K-H, Höfle G, Müller R, Mulzer J, Prantz K. The Epothilones: an Outstanding Family of Anti-Tumor Agents – From Soil to the Clinic. Prog Chem Org Nat Prod. 2009;90:1. - PubMed
-
- Balunas MJ, Jones WP, Chin Y-W, Mi Q, Farnsworth NR, Soejarto DD, Cordell GA, Swanson SM, Pezzuto JM, Chai H-B, Kinghorn AD. Relationships between Inhibitory Activity against a Cancer Cell Line Panel, Profiles of Plants Collected, and Compound Classes Isolated in an Anticancer Drug Discovery Project. Chem Biodiversity. 2006;3:897. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
