Cytokine signaling modulates blood-brain barrier function
- PMID: 21834767
- PMCID: PMC3939622
- DOI: 10.2174/138161211798220918
Cytokine signaling modulates blood-brain barrier function
Abstract
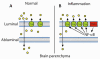
The blood-brain barrier (BBB) provides a vast interface for cytokines to affect CNS function. The BBB is a target for therapeutic intervention. It is essential, therefore, to understand how cytokines interact with each other at the level of the BBB and how secondary signals modulate CNS functions beyond the BBB. The interactions between cytokines and lipids, however, have not been fully addressed at the level of the BBB. Here, we summarize current understanding of the localization of cytokine receptors and transporters in specific membrane microdomains, particularly lipid rafts, on the luminal (apical) surface of the microvascular endothelial cells composing the BBB. We then illustrate the clinical context of cytokine effects on the BBB by neuroendocrine regulation and amplification of inflammatory signals. Two unusual aspects discussed are signaling crosstalk by different classes of cytokines and genetic regulation of drug efflux transporters. We also introduce a novel area of focus on how cytokines may act through nuclear hormone receptors to modulate efflux transporters and other targets. A specific example discussed is the ATP-binding cassette transporter-1 (ABCA-1) that regulates lipid metabolism. Overall, cytokine signaling at the level of the BBB is a crucial feature of the dynamic regulation that can rapidly change BBB function and affect brain health and disease.
Figures
References
-
- Begley DJ. Peptides and the blood-brain barrier: the status of our understanding. Ann NY Acad Sci. 1994;739:89–100. - PubMed
-
- Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ. Structure and function of the blood-brain barrier. Neurobiol Dis. 2010;37(1):13–25. - PubMed
-
- Neuwelt EA. Mechanisms of disease: the blood-brain barrier. Neurosurgery. 2004;54(1):131–40. - PubMed
-
- Minn A, Ghersi-Egea JF, Perrin R, Leininger B, Siest G. Drug metabolizing enzymes in the brain and cerebral microvessels. Brain Res Brain Res Rev. 1991;16(1):65–82. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

