Uptake and fate of surface modified silica nanoparticles in head and neck squamous cell carcinoma
- PMID: 21834958
- PMCID: PMC3164619
- DOI: 10.1186/1477-3155-9-32
Uptake and fate of surface modified silica nanoparticles in head and neck squamous cell carcinoma
Abstract
Background: Head and neck squamous cell carcinoma (HNSCC) is currently the eighth leading cause of cancer death worldwide. The often severe side effects, functional impairments and unfavorable cosmetic outcome of conventional therapies for HNSCC have prompted the quest for novel treatment strategies, including the evaluation of nanotechnology to improve e.g. drug delivery and cancer imaging. Although silica nanoparticles hold great promise for biomedical applications, they have not yet been investigated in the context of HNSCC. In the present in-vitro study we thus analyzed the cytotoxicity, uptake and intracellular fate of 200-300 nm core-shell silica nanoparticles encapsulating fluorescent dye tris(bipyridine)ruthenium(II) dichloride with hydroxyl-, aminopropyl- or PEGylated surface modifications (Ru@SiO2-OH, Ru@SiO2-NH2, Ru@SiO2-PEG) in the human HNSCC cell line UMB-SCC 745.
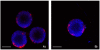
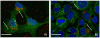
Results: We found that at concentrations of 0.125 mg/ml, none of the nanoparticles used had a statistically significant effect on proliferation rates of UMB-SCC 745. Confocal and transmission electron microscopy showed an intracellular appearance of Ru@SiO2-OH and Ru@SiO2-NH2 within 30 min. They were internalized both as single nanoparticles (presumably via clathrin-coated pits) or in clusters and always localized to cytoplasmic membrane-bounded vesicles. Immunocytochemical co-localization studies indicated that only a fraction of these nanoparticles were transferred to early endosomes, while the majority accumulated in large organelles. Ru@SiO2-OH and Ru@SiO2-NH2 nanoparticles had never been observed to traffic to the lysosomal compartment and were rather propagated at cell division. Intracellular persistence of Ru@SiO2-OH and Ru@SiO2-NH2 was thus traceable over 5 cell passages, but did not result in apparent changes in cell morphology and vitality. In contrast to Ru@SiO2-OH and Ru@SiO2-NH2 uptake of Ru@SiO2-PEG was minimal even after 24 h.
Conclusions: Our study is the first to provide evidence that silica-based nanoparticles may serve as useful tools for the development of novel treatment options in HNSCC. Their long intracellular persistence could be of advantage for e.g. chronic therapeutic modalities. However, their complex endocytotic pathways require further investigations.
Figures
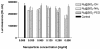







References
-
- Vermorken JB, Specenier P. Optimal treatment for recurrent/metastatic head and neck cancer. Ann Oncol. pp. vii252–vii261. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

