A pilot study to evaluate the application of a generic protein standard panel for quality control of biomarker detection technologies
- PMID: 21834984
- PMCID: PMC3162916
- DOI: 10.1186/1756-0500-4-281
A pilot study to evaluate the application of a generic protein standard panel for quality control of biomarker detection technologies
Abstract
Background: Protein biomarker studies are currently hampered by a lack of measurement standards to demonstrate quality, reliability and comparability across multiple assay platforms. This is especially pertinent for immunoassays where multiple formats for detecting target analytes are commonly used.
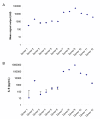
Findings: In this pilot study a generic panel of six non-human protein standards (50 - 10^7 pg/mL) of varying abundance was prepared as a quality control (QC) material. Simulated "normal" and "diseased" panels of proteins were prepared in pooled human plasma and incorporated into immunoassays using the Meso Scale Discovery® (MSD®) platform to illustrate reliable detection of the component proteins. The protein panel was also evaluated as a spike-in material for a model immunoassay involving detection of ovarian cancer biomarkers within individual human plasma samples. Our selected platform could discriminate between two panels of the proteins exhibiting small differences in abundance. Across distinct experiments, all component proteins exhibited reproducible signal outputs in pooled human plasma. When individual donor samples were used, half the proteins produced signals independent of matrix effects. These proteins may serve as a generic indicator of platform reliability.Each of the remaining proteins exhibit differential signals across the distinct samples, indicative of sample matrix effects, with the three proteins following the same trend. This subset of proteins may be useful for characterising the degree of matrix effects associated with the sample which may impact on the reliability of quantifying target diagnostic biomarkers.
Conclusions: We have demonstrated the potential utility of this panel of standards to act as a generic QC tool for evaluating the reproducibility of the platform for protein biomarker detection independent of serum matrix effects.
Figures


Similar articles
-
Cross-platform comparison of highly sensitive immunoassay technologies for cytokine markers: Platform performance in post-traumatic stress disorder and Parkinson's disease.Cytokine X. 2020 Apr 28;2(2):100027. doi: 10.1016/j.cytox.2020.100027. eCollection 2020 Jun. Cytokine X. 2020. PMID: 33604555 Free PMC article.
-
Evaluation of external RNA controls for the standardisation of gene expression biomarker measurements.BMC Genomics. 2010 Nov 24;11:662. doi: 10.1186/1471-2164-11-662. BMC Genomics. 2010. PMID: 21106083 Free PMC article.
-
Comparison of Bead-Based Fluorescence Versus Planar Electrochemiluminescence Multiplex Immunoassays for Measuring Cytokines in Human Plasma.Front Immunol. 2020 Sep 24;11:572634. doi: 10.3389/fimmu.2020.572634. eCollection 2020. Front Immunol. 2020. PMID: 33101295 Free PMC article.
-
Optomechanical devices for deep plasma cancer proteomics.Semin Cancer Biol. 2018 Oct;52(Pt 1):26-38. doi: 10.1016/j.semcancer.2017.08.011. Epub 2017 Sep 1. Semin Cancer Biol. 2018. PMID: 28867489 Review.
-
Protocol for Standardizing High-to-Moderate Abundance Protein Biomarker Assessments Through an MRM-with-Standard-Peptides Quantitative Approach.Adv Exp Med Biol. 2016;919:515-530. doi: 10.1007/978-3-319-41448-5_24. Adv Exp Med Biol. 2016. PMID: 27975233 Review.
References
-
- US Food and Drug Administration. Challenge and Opportunity on the Critical Path to New Medicinal Products. USA; 2004. http://www.who.int/intellectualproperty/documents/en/FDAproposals.pdf
LinkOut - more resources
Full Text Sources

