Diffusion kurtosis as an in vivo imaging marker for reactive astrogliosis in traumatic brain injury
- PMID: 21835250
- PMCID: PMC3614502
- DOI: 10.1016/j.neuroimage.2011.07.050
Diffusion kurtosis as an in vivo imaging marker for reactive astrogliosis in traumatic brain injury
Abstract
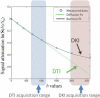
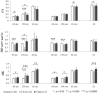
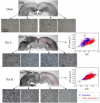
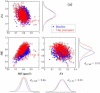
Diffusion Kurtosis Imaging (DKI) provides quantifiable information on the non-Gaussian behavior of water diffusion in biological tissue. Changes in water diffusion tensor imaging (DTI) parameters and DKI parameters in several white and gray matter regions were investigated in a mild controlled cortical impact (CCI) injury rat model at both the acute (2 h) and the sub-acute (7 days) stages following injury. Mixed model ANOVA analysis revealed significant changes in temporal patterns of both DTI and DKI parameters in the cortex, hippocampus, external capsule and corpus callosum. Post-hoc tests indicated acute changes in mean diffusivity (MD) in the bilateral cortex and hippocampus (p<0.0005) and fractional anisotropy (FA) in ipsilateral cortex (p<0.0005), hippocampus (p=0.014), corpus callosum (p=0.031) and contralateral external capsule (p=0.011). These changes returned to baseline by the sub-acute stage. However, mean kurtosis (MK) was significantly elevated at the sub-acute stages in all ipsilateral regions and scaled inversely with the distance from the impacted site (cortex and corpus callosum: p<0.0005; external capsule: p=0.003; hippocampus: p=0.011). Further, at the sub-acute stage increased MK was also observed in the contralateral regions compared to baseline (cortex: p=0.032; hippocampus: p=0.039) while no change was observed with MD and FA. An increase in mean kurtosis was associated with increased reactive astrogliosis from immunohistochemistry analysis. Our results suggest that DKI is sensitive to microstructural changes associated with reactive astrogliosis which may be missed by standard DTI parameters alone. Monitoring changes in MK allows the investigation of molecular and morphological changes in vivo due to reactive astrogliosis and may complement information available from standard DTI parameters. To date the use of diffusion tensor imaging has been limited to study changes in white matter integrity following traumatic insults. Given the sensitivity of DKI to detect microstructural changes even in the gray matter in vivo, allows the extension of the technique to understand patho-morphological changes in the whole brain following a traumatic insult.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures




Similar articles
-
Dynamics of blood brain barrier permeability and tissue microstructure following controlled cortical impact injury in rat: A dynamic contrast-enhanced magnetic resonance imaging and diffusion kurtosis imaging study.Magn Reson Imaging. 2019 Oct;62:1-9. doi: 10.1016/j.mri.2019.01.017. Epub 2019 Jan 17. Magn Reson Imaging. 2019. PMID: 30660704
-
Exploring IVIM-DKI and DKI for Assessing Microvascular and Microstructural Changes After Traumatic Brain Injury.NMR Biomed. 2025 Sep;38(9):e70110. doi: 10.1002/nbm.70110. NMR Biomed. 2025. PMID: 40751360
-
Can diffusion kurtosis imaging improve the sensitivity and specificity of detecting microstructural alterations in brain tissue chronically after experimental stroke? Comparisons with diffusion tensor imaging and histology.Neuroimage. 2014 Aug 15;97:363-73. doi: 10.1016/j.neuroimage.2014.04.013. Epub 2014 Apr 15. Neuroimage. 2014. PMID: 24742916
-
Differences in Gaussian diffusion tensor imaging and non-Gaussian diffusion kurtosis imaging model-based estimates of diffusion tensor invariants in the human brain.Med Phys. 2016 May;43(5):2464. doi: 10.1118/1.4946819. Med Phys. 2016. PMID: 27147357
-
The role of diffusion tensor imaging and fractional anisotropy in the evaluation of patients with idiopathic normal pressure hydrocephalus: a literature review.Neurosurg Focus. 2016 Sep;41(3):E12. doi: 10.3171/2016.6.FOCUS16192. Neurosurg Focus. 2016. PMID: 27581308 Review.
Cited by
-
Abnormalities in Brain and Muscle Microstructure and Neurochemistry of the DMD Rat Measured by in vivo Diffusion Tensor Imaging and High Resolution Localized 1H MRS.Front Neurosci. 2020 Jul 14;14:739. doi: 10.3389/fnins.2020.00739. eCollection 2020. Front Neurosci. 2020. PMID: 32760246 Free PMC article.
-
Diffusional kurtosis imaging of normal-appearing white matter in multiple sclerosis: preliminary clinical experience.Jpn J Radiol. 2013 Jan;31(1):50-5. doi: 10.1007/s11604-012-0147-7. Epub 2012 Oct 23. Jpn J Radiol. 2013. PMID: 23086313
-
Attention-deficit/hyperactivity disorder without comorbidity is associated with distinct atypical patterns of cerebral microstructural development.Hum Brain Mapp. 2014 May;35(5):2148-62. doi: 10.1002/hbm.22317. Epub 2013 Aug 1. Hum Brain Mapp. 2014. PMID: 23907808 Free PMC article.
-
A preliminary study of epilepsy in children using diffusional kurtosis imaging.Clin Neuroradiol. 2013 Dec;23(4):293-300. doi: 10.1007/s00062-013-0212-3. Epub 2013 May 29. Clin Neuroradiol. 2013. PMID: 23715877
-
Neurobiochemical, Peptidomic, and Bioinformatic Approaches to Characterize Tauopathy Peptidome Biomarker Candidates in Experimental Mouse Model of Traumatic Brain Injury.Mol Neurobiol. 2023 Apr;60(4):2295-2319. doi: 10.1007/s12035-022-03165-y. Epub 2023 Jan 13. Mol Neurobiol. 2023. PMID: 36635478
References
-
- Armitage PA, Bastin ME, Marshall I, Wardlaw JM, Cannon J. Diffusion anisotropy measurements in ischaemic stroke of the human brain. MAGMA. 1998;6(1):28–36. - PubMed
-
- Assaf Y, Cohen Y. Non-mono-exponential attenuation of water and N-acetyl aspartate signals due to diffusion in brain tissue. J. Magn. Reson. 1998;131(1):69–85. - PubMed
-
- Assaf Y, Cohen Y. Assignment of the water slow-diffusing component in the central nervous system using q-space diffusion MRS: implications for fiber tract imaging. Magn Reson Med. 2000;43:191–199. - PubMed
-
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Statist. Soc. B. 1995;57(1):289–300.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

