Comparisons between murine polyomavirus and Simian virus 40 show significant differences in small T antigen function
- PMID: 21835797
- PMCID: PMC3187521
- DOI: 10.1128/JVI.05034-11
Comparisons between murine polyomavirus and Simian virus 40 show significant differences in small T antigen function
Abstract
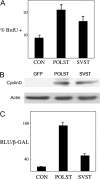
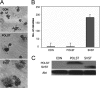
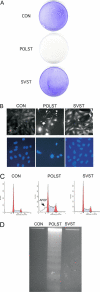
Although members of a virus family produce similar gene products, those products may have quite different functions. Simian virus 40 (SV40) large T antigen (LT), for example, targets p53 directly, but murine polyomavirus LT does not. SV40 small T antigen (SVST) has received considerable attention because of its ability to contribute to transformation of human cells. Here, we show that there are major differences between SVST and polyomavirus small T antigen (POLST) in their effects on differentiation, transformation, and cell survival. Both SVST and POLST induce cell cycle progression. However, POLST also inhibits differentiation of 3T3-L1 preadipocytes and C2C12 myoblasts. Additionally, POLST induces apoptosis of mouse embryo fibroblasts. SVST reduces the proapoptotic transcriptional activity of FOXO1 through phosphorylation. On the other hand, SVST complements large T antigen and Ras for the transformation of human mammary epithelial cells (HMECs), but POLST does not. Mechanistically, the differences between SVST and POLST may lie in utilization of protein phosphatase 2A (PP2A). POLST binds both Aα and Aβ scaffolding subunits of PP2A while SVST binds only Aα. Knockdown of Aβ could mimic POLST-induced apoptosis. The two small T antigens can target different proteins for dephosphorylation. POLST binds and dephosphorylates substrates, such as lipins, that SVST does not.
Figures






Similar articles
-
Polyomavirus Small T Antigen Induces Apoptosis in Mammalian Cells through the UNC5B Pathway in a PP2A-Dependent Manner.J Virol. 2020 Jul 1;94(14):e02187-19. doi: 10.1128/JVI.02187-19. Print 2020 Jul 1. J Virol. 2020. PMID: 32404521 Free PMC article.
-
Restricted protein phosphatase 2A targeting by Merkel cell polyomavirus small T antigen.J Virol. 2015 Apr;89(8):4191-200. doi: 10.1128/JVI.00157-15. Epub 2015 Jan 28. J Virol. 2015. PMID: 25631078 Free PMC article.
-
Cellular transformation by Simian Virus 40 and Murine Polyoma Virus T antigens.Semin Cancer Biol. 2009 Aug;19(4):218-28. doi: 10.1016/j.semcancer.2009.03.002. Epub 2009 Mar 31. Semin Cancer Biol. 2009. PMID: 19505649 Free PMC article. Review.
-
Protein phosphatase 2A isoforms utilizing Aβ scaffolds regulate differentiation through control of Akt protein.J Biol Chem. 2013 Nov 1;288(44):32064-73. doi: 10.1074/jbc.M113.497644. Epub 2013 Sep 19. J Biol Chem. 2013. PMID: 24052256 Free PMC article.
-
Involvement of PP2A in viral and cellular transformation.Oncogene. 2005 Nov 21;24(52):7746-55. doi: 10.1038/sj.onc.1209038. Oncogene. 2005. PMID: 16299534 Review.
Cited by
-
Papillomavirus E7 oncoproteins share functions with polyomavirus small T antigens.J Virol. 2015 Mar;89(5):2857-65. doi: 10.1128/JVI.03282-14. Epub 2014 Dec 24. J Virol. 2015. PMID: 25540383 Free PMC article.
-
A degradable form of polyoma small T antigen reveals the high specificity of TAZ in regulating gene expression.Proc Natl Acad Sci U S A. 2025 Jul 8;122(27):e2426862122. doi: 10.1073/pnas.2426862122. Epub 2025 Jul 3. Proc Natl Acad Sci U S A. 2025. PMID: 40608673
-
Transformation by Polyomavirus Middle T Antigen Involves a Unique Bimodal Interaction with the Hippo Effector YAP.J Virol. 2016 Jul 27;90(16):7032-7045. doi: 10.1128/JVI.00417-16. Print 2016 Aug 15. J Virol. 2016. PMID: 27194756 Free PMC article.
-
Polyomavirus interaction with the DNA damage response.Virol Sin. 2015 Apr;30(2):122-9. doi: 10.1007/s12250-015-3583-6. Epub 2015 Apr 20. Virol Sin. 2015. PMID: 25910481 Free PMC article. Review.
-
Polyomavirus Small T Antigen Induces Apoptosis in Mammalian Cells through the UNC5B Pathway in a PP2A-Dependent Manner.J Virol. 2020 Jul 1;94(14):e02187-19. doi: 10.1128/JVI.02187-19. Print 2020 Jul 1. J Virol. 2020. PMID: 32404521 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

