A high content drug screen identifies ursolic acid as an inhibitor of amyloid beta protein interactions with its receptor CD36
- PMID: 21835916
- PMCID: PMC3186388
- DOI: 10.1074/jbc.M111.232116
A high content drug screen identifies ursolic acid as an inhibitor of amyloid beta protein interactions with its receptor CD36
Abstract
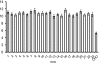
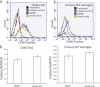
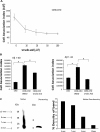
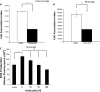
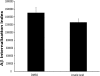
A pathological hallmark of Alzheimer disease (AD) is deposition of amyloid β (Aβ) in the brain. Aβ binds to microglia via a receptor complex that includes CD36 leading to production of proinflammatory cytokines and neurotoxic reactive oxygen species and subsequent neurodegeneration. Interruption of Aβ binding to CD36 is a potential therapeutic strategy for AD. To identify pharmacologic inhibitors of Aβ binding to CD36, we developed a 384-well plate assay for binding of fluorescently labeled Aβ to Chinese hamster ovary cells stably expressing human CD36 (CHO-CD36) and screened an Food and Drug Administration-approved compound library. The assay was optimized based on the cells' tolerance to dimethyl sulfoxide, Aβ concentration, time required for Aβ binding, reproducibility, and signal-to-background ratio. Using this assay, we identified four compounds as potential inhibitors of Aβ binding to CD36. These compounds were ursolic acid, ellipticine, zoxazolamine, and homomoschatoline. Of these compounds, only ursolic acid, a naturally occurring pentacyclic triterpenoid, successfully inhibited binding of Aβ to CHO-CD36 cells in a dose-dependent manner. The ursolic acid effect reached a plateau at ~20 μm, with a maximal inhibition of 64%. Ursolic acid also blocked binding of Aβ to microglial cells and subsequent ROS production. Our data indicate that cell-based high-content screening of small molecule libraries for their ability to block binding of Aβ to its receptors is a useful tool to identify novel inhibitors of receptors involved in AD pathogenesis. Our data also suggest that ursolic acid is a potential therapeutic agent for AD via its ability to block Aβ-CD36 interactions.
Figures

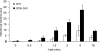
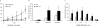
Similar articles
-
IL-4-induced selective clearance of oligomeric beta-amyloid peptide(1-42) by rat primary type 2 microglia.J Immunol. 2008 Nov 1;181(9):6503-13. doi: 10.4049/jimmunol.181.9.6503. J Immunol. 2008. PMID: 18941241
-
A cell surface receptor complex for fibrillar beta-amyloid mediates microglial activation.J Neurosci. 2003 Apr 1;23(7):2665-74. doi: 10.1523/JNEUROSCI.23-07-02665.2003. J Neurosci. 2003. PMID: 12684452 Free PMC article.
-
Prostaglandin E2 receptor subtype 2 regulation of scavenger receptor CD36 modulates microglial Aβ42 phagocytosis.Am J Pathol. 2015 Jan;185(1):230-9. doi: 10.1016/j.ajpath.2014.09.016. Epub 2014 Nov 15. Am J Pathol. 2015. PMID: 25452117 Free PMC article.
-
Microglial Aβ receptors in Alzheimer's disease.Cell Mol Neurobiol. 2015 Jan;35(1):71-83. doi: 10.1007/s10571-014-0101-6. Epub 2014 Aug 23. Cell Mol Neurobiol. 2015. PMID: 25149075 Free PMC article. Review.
-
Binding sites of amyloid beta-peptide in cell plasma membrane and implications for Alzheimer's disease.Curr Protein Pept Sci. 2004 Feb;5(1):19-31. doi: 10.2174/1389203043486937. Curr Protein Pept Sci. 2004. PMID: 14965318 Review.
Cited by
-
The Past and Present Lives of the Intraocular Transmembrane Protein CD36.Cells. 2022 Dec 31;12(1):171. doi: 10.3390/cells12010171. Cells. 2022. PMID: 36611964 Free PMC article. Review.
-
Microglia as a critical player in both developmental and late-life CNS pathologies.Acta Neuropathol. 2014 Sep;128(3):333-45. doi: 10.1007/s00401-014-1321-z. Epub 2014 Jul 24. Acta Neuropathol. 2014. PMID: 25056803 Free PMC article. Review.
-
Combined Ursolic Acid and Resistance/Endurance Training Improve Type 3 Diabetes Biomarkers-Related Memory Deficits in Hippocampus of Aged Male Wistar Rats.Int J Prev Med. 2023 May 27;14:65. doi: 10.4103/ijpvm.ijpvm_317_21. eCollection 2023. Int J Prev Med. 2023. PMID: 37351031 Free PMC article.
-
Molecular and genetic inflammation networks in major human diseases.Mol Biosyst. 2016 Jul 19;12(8):2318-41. doi: 10.1039/c6mb00240d. Mol Biosyst. 2016. PMID: 27303926 Free PMC article. Review.
-
Microglia receptors and their implications in the response to amyloid β for Alzheimer's disease pathogenesis.J Neuroinflammation. 2014 Mar 13;11:48. doi: 10.1186/1742-2094-11-48. J Neuroinflammation. 2014. PMID: 24625061 Free PMC article. Review.
References
-
- Grant W. B. (2004) Arch. Neurol. 61, 802-a-803 - PubMed
-
- Selkoe D. J. (2000) JAMA 283, 1615–1617 - PubMed
-
- Akiyama H., Barger S., Barnum S., Bradt B., Bauer J., Cole G. M., Cooper N. R., Eikelenboom P., Emmerling M., Fiebich B. L., Finch C. E., Frautschy S., Griffin W. S., Hampel H., Hull M., Landreth G., Lue L., Mrak R., Mackenzie I. R., McGeer P. L., O'Banion M. K., Pachter J., Pasinetti G., Plata-Salaman C., Rogers J., Rydel R., Shen Y., Streit W., Strohmeyer R., Tooyoma I., Van Muiswinkel F. L., Veerhuis R., Walker D., Webster S., Wegrzyniak B., Wenk G., Wyss-Coray T. (2000) Neurobiol. Aging 21, 383–421 - PMC - PubMed
-
- Ricciarelli R., D'Abramo C., Zingg J. M., Giliberto L., Markesbery W., Azzi A., Marinari U. M., Pronzato M. A., Tabaton M. (2004) Free Radic. Biol. Med. 36, 1018–1024 - PubMed
-
- Itagaki S., McGeer P. L., Akiyama H., Zhu S., Selkoe D. (1989) J. Neuroimmunol. 24, 173–182 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

