Ribosome assembly factors prevent premature translation initiation by 40S assembly intermediates
- PMID: 21835981
- PMCID: PMC3402165
- DOI: 10.1126/science.1208245
Ribosome assembly factors prevent premature translation initiation by 40S assembly intermediates
Abstract
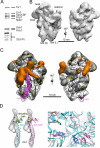
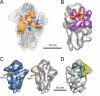
Ribosome assembly in eukaryotes requires approximately 200 essential assembly factors (AFs) and occurs through ordered events that initiate in the nucleolus and culminate in the cytoplasm. Here, we present the electron cryo-microscopy (cryo-EM) structure of a late cytoplasmic 40S ribosome assembly intermediate from Saccharomyces cerevisiae at 18 angstrom resolution. We obtained cryo-EM reconstructions of preribosomal complexes lacking individual components to define the positions of all seven AFs bound to this intermediate. These late-binding AFs are positioned to prevent each step in the translation initiation pathway. Together, they obstruct the binding sites for initiation factors, prevent the opening of the messenger RNA channel, block 60S subunit joining, and disrupt the decoding site. These redundant mechanisms probably ensure that pre-40S particles do not enter the translation pathway, which would result in their rapid degradation.
Figures


Similar articles
-
Immature small ribosomal subunits can engage in translation initiation in Saccharomyces cerevisiae.EMBO J. 2010 Jan 6;29(1):80-92. doi: 10.1038/emboj.2009.307. Epub 2009 Nov 5. EMBO J. 2010. PMID: 19893492 Free PMC article.
-
An open interface in the pre-80S ribosome coordinated by ribosome assembly factors Tsr1 and Dim1 enables temporal regulation of Fap7.RNA. 2021 Feb;27(2):221-233. doi: 10.1261/rna.077610.120. Epub 2020 Nov 20. RNA. 2021. PMID: 33219089 Free PMC article.
-
Molecular architecture of the 40S⋅eIF1⋅eIF3 translation initiation complex.Cell. 2014 Aug 28;158(5):1123-1135. doi: 10.1016/j.cell.2014.07.044. Cell. 2014. PMID: 25171412 Free PMC article.
-
Inside the 40S ribosome assembly machinery.Curr Opin Chem Biol. 2011 Oct;15(5):657-63. doi: 10.1016/j.cbpa.2011.07.023. Epub 2011 Aug 20. Curr Opin Chem Biol. 2011. PMID: 21862385 Free PMC article. Review.
-
Eukaryotic Ribosome Assembly.Annu Rev Biochem. 2024 Aug;93(1):189-210. doi: 10.1146/annurev-biochem-030222-113611. Epub 2024 Jul 2. Annu Rev Biochem. 2024. PMID: 38768392 Review.
Cited by
-
Surveillance pathways rescuing eukaryotic ribosomes lost in translation.Nat Rev Mol Cell Biol. 2012 Nov;13(11):727-35. doi: 10.1038/nrm3457. Epub 2012 Oct 17. Nat Rev Mol Cell Biol. 2012. PMID: 23072885 Review.
-
Co-translational capturing of nascent ribosomal proteins by their dedicated chaperones.Nat Commun. 2015 Jun 26;6:7494. doi: 10.1038/ncomms8494. Nat Commun. 2015. PMID: 26112308 Free PMC article.
-
Role of Era in assembly and homeostasis of the ribosomal small subunit.Nucleic Acids Res. 2019 Sep 5;47(15):8301-8317. doi: 10.1093/nar/gkz571. Nucleic Acids Res. 2019. PMID: 31265110 Free PMC article.
-
Knockdown of NOB1 expression inhibits the malignant transformation of human prostate cancer cells.Mol Cell Biochem. 2014 Nov;396(1-2):1-8. doi: 10.1007/s11010-014-2126-z. Epub 2014 Aug 29. Mol Cell Biochem. 2014. PMID: 25169742
-
Interaction network of the ribosome assembly machinery from a eukaryotic thermophile.Protein Sci. 2017 Feb;26(2):327-342. doi: 10.1002/pro.3085. Epub 2017 Jan 14. Protein Sci. 2017. PMID: 27863450 Free PMC article.
References
-
- Frank J, et al. A model of protein synthesis based on cryo-electron microscopy of the E. coli ribosome. Nature. 376:441. - PubMed
-
- Beckmann R, et al. Alignment of conduits for the nascent polypeptide chain in the ribosome-Sec61 complex. Science. 278:2123. - PubMed
-
- Spahn CM, et al. Structure of the 80S ribosome from Saccharomyces cerevisiae--tRNA-ribosome and subunit-subunit interactions. Cell. 107:373. - PubMed
-
- Spahn CM, et al. Cryo-EM visualization of a viral internal ribosome entry site bound to human ribosomes: the IRES functions as an RNA-based translation factor. Cell. 118:465. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

