The structure of the kinesin-1 motor-tail complex reveals the mechanism of autoinhibition
- PMID: 21836017
- PMCID: PMC3339660
- DOI: 10.1126/science.1204824
The structure of the kinesin-1 motor-tail complex reveals the mechanism of autoinhibition
Abstract
When not transporting cargo, kinesin-1 is autoinhibited by binding of a tail region to the motor domains, but the mechanism of inhibition is unclear. We report the crystal structure of a motor domain dimer in complex with its tail domain at 2.2 angstroms and compare it with a structure of the motor domain alone at 2.7 angstroms. These structures indicate that neither an induced conformational change nor steric blocking is the cause of inhibition. Instead, the tail cross-links the motor domains at a second position, in addition to the coiled coil. This "double lockdown," by cross-linking at two positions, prevents the movement of the motor domains that is needed to undock the neck linker and release adenosine diphosphate. This autoinhibition mechanism could extend to some other kinesins.
Figures
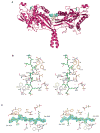

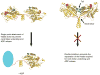
Similar articles
-
Half-site inhibition of dimeric kinesin head domains by monomeric tail domains.Biochemistry. 2009 Apr 21;48(15):3448-56. doi: 10.1021/bi8022575. Biochemistry. 2009. PMID: 19320433 Free PMC article.
-
A kinesin motor in a force-producing conformation.BMC Struct Biol. 2010 Jul 5;10:19. doi: 10.1186/1472-6807-10-19. BMC Struct Biol. 2010. PMID: 20602775 Free PMC article.
-
A metal switch for controlling the activity of molecular motor proteins.Nat Struct Mol Biol. 2011 Dec 25;19(1):122-7. doi: 10.1038/nsmb.2190. Nat Struct Mol Biol. 2011. PMID: 22198464 Free PMC article.
-
Structural links to kinesin directionality and movement.Nat Struct Biol. 2000 Jun;7(6):456-60. doi: 10.1038/75850. Nat Struct Biol. 2000. PMID: 10881190 Review.
-
Kinesin: switch I & II and the motor mechanism.J Cell Sci. 2002 Jan 1;115(Pt 1):15-23. doi: 10.1242/jcs.115.1.15. J Cell Sci. 2002. PMID: 11801720 Review.
Cited by
-
Functional asymmetry in kinesin and dynein dimers.Biol Cell. 2013 Jan;105(1):1-13. doi: 10.1111/boc.201200044. Epub 2012 Dec 5. Biol Cell. 2013. PMID: 23066835 Free PMC article. Review.
-
A chimeric kinesin-1 head/kinesin-5 tail motor switches between diffusive and processive motility.Biophys J. 2013 Jan 22;104(2):432-41. doi: 10.1016/j.bpj.2012.11.3810. Biophys J. 2013. PMID: 23442865 Free PMC article.
-
Biased Brownian motion as a mechanism to facilitate nanometer-scale exploration of the microtubule plus end by a kinesin-8.Proc Natl Acad Sci U S A. 2015 Jul 21;112(29):E3826-35. doi: 10.1073/pnas.1500272112. Epub 2015 Jul 6. Proc Natl Acad Sci U S A. 2015. PMID: 26150501 Free PMC article.
-
Single molecule FRET observation of kinesin-1's head-tail interaction on microtubule.Biophysics (Nagoya-shi). 2013 Nov 7;9:149-59. doi: 10.2142/biophysics.9.149. eCollection 2013. Biophysics (Nagoya-shi). 2013. PMID: 27493553 Free PMC article.
-
Simulations suggest robust microtubule attachment of kinesin and dynein in antagonistic pairs.Biophys J. 2023 Aug 22;122(16):3299-3313. doi: 10.1016/j.bpj.2023.07.007. Epub 2023 Jul 17. Biophys J. 2023. PMID: 37464742 Free PMC article.
References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

