Small-molecule activators of TMEM16A, a calcium-activated chloride channel, stimulate epithelial chloride secretion and intestinal contraction
- PMID: 21836025
- PMCID: PMC3205834
- DOI: 10.1096/fj.11-191627
Small-molecule activators of TMEM16A, a calcium-activated chloride channel, stimulate epithelial chloride secretion and intestinal contraction
Abstract
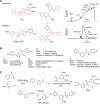
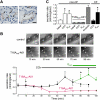
TMEM16A (ANO1) is a calcium-activated chloride channel (CaCC) expressed in secretory epithelia, smooth muscle, and other tissues. Cell-based functional screening of ∼110,000 compounds revealed compounds that activated TMEM16A CaCC conductance without increasing cytoplasmic Ca(2+). By patch-clamp, N-aroylaminothiazole "activators" (E(act)) strongly increased Cl(-) current at 0 Ca(2+), whereas tetrazolylbenzamide "potentiators" (F(act)) were not active at 0 Ca(2+) but reduced the EC(50) for Ca(2+)-dependent TMEM16A activation. Of 682 analogs tested, the most potent activator (E(act)) and potentiator (F(act)) produced large and more sustained CaCC Cl(-) currents than general agonists of Ca(2+) signaling, with EC(50) 3-6 μM and Cl(-) conductance comparable to that induced transiently by Ca(2+)-elevating purinergic agonists. Analogs of activators were identified that fully inhibited TMEM16A Cl(-) conductance, providing further evidence for direct TMEM16A binding. The TMEM16A activators increased CaCC conductance in human salivary and airway submucosal gland epithelial cells, and IL-4 treated bronchial cells, and stimulated submucosal gland secretion in human bronchi and smooth muscle contraction in mouse intestine. Small-molecule, TMEM16A-targeted activators may be useful for drug therapy of cystic fibrosis, dry mouth, and gastrointestinal hypomotility disorders, and for pharmacological dissection of TMEM16A function.
Figures




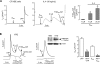
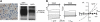

Similar articles
-
TMEM16A inhibitors reveal TMEM16A as a minor component of calcium-activated chloride channel conductance in airway and intestinal epithelial cells.J Biol Chem. 2011 Jan 21;286(3):2365-74. doi: 10.1074/jbc.M110.175109. Epub 2010 Nov 17. J Biol Chem. 2011. PMID: 21084298 Free PMC article.
-
Control of TMEM16A by INO-4995 and other inositolphosphates.Br J Pharmacol. 2013 Jan;168(1):253-65. doi: 10.1111/j.1476-5381.2012.02193.x. Br J Pharmacol. 2013. PMID: 22946960 Free PMC article.
-
Revealing the activation pathway for TMEM16A chloride channels from macroscopic currents and kinetic models.Pflugers Arch. 2016 Jul;468(7):1241-1257. doi: 10.1007/s00424-016-1830-9. Epub 2016 May 2. Pflugers Arch. 2016. PMID: 27138167 Free PMC article.
-
Calcium-Activated Cl- Channel: Insights on the Molecular Identity in Epithelial Tissues.Int J Mol Sci. 2018 May 10;19(5):1432. doi: 10.3390/ijms19051432. Int J Mol Sci. 2018. PMID: 29748496 Free PMC article. Review.
-
Bestrophin and TMEM16-Ca(2+) activated Cl(-) channels with different functions.Cell Calcium. 2009 Oct;46(4):233-41. doi: 10.1016/j.ceca.2009.09.003. Epub 2009 Sep 26. Cell Calcium. 2009. PMID: 19783045 Review.
Cited by
-
TMEM16A Potentiation: A Novel Therapeutic Approach for the Treatment of Cystic Fibrosis.Am J Respir Crit Care Med. 2020 Apr 15;201(8):946-954. doi: 10.1164/rccm.201908-1641OC. Am J Respir Crit Care Med. 2020. PMID: 31898911 Free PMC article.
-
The Natural Compound Cinnamaldehyde is a Novel Activator of Calcium-Activated Chloride Channel.J Membr Biol. 2018 Dec;251(5-6):747-756. doi: 10.1007/s00232-018-0052-9. Epub 2018 Oct 31. J Membr Biol. 2018. PMID: 30382294
-
Recent advances in developing therapeutics for cystic fibrosis.Hum Mol Genet. 2018 Aug 1;27(R2):R173-R186. doi: 10.1093/hmg/ddy188. Hum Mol Genet. 2018. PMID: 30060192 Free PMC article. Review.
-
VI-116, A Novel Potent Inhibitor of VRAC with Minimal Effect on ANO1.Int J Mol Sci. 2022 May 5;23(9):5168. doi: 10.3390/ijms23095168. Int J Mol Sci. 2022. PMID: 35563558 Free PMC article.
-
Pharmacological Modulation of Ion Channels for the Treatment of Cystic Fibrosis.J Exp Pharmacol. 2021 Jul 23;13:693-723. doi: 10.2147/JEP.S255377. eCollection 2021. J Exp Pharmacol. 2021. PMID: 34326672 Free PMC article. Review.
References
-
- Hartzell C., Putzier I., Arreola J. (2005) Calcium-activated chloride channels. Annu. Rev. Physiol. 67, 719–758 - PubMed
-
- Eggermont J. (2004) Calcium-activated chloride channels: (un) known, (un)loved? Proc. Am. Thorac. Soc. 1, 22–27 - PubMed
-
- Yang Y. D., Cho H., Koo J. Y., Tak M. H., Cho Y., Shim W. S., Park S. P., Lee J., Lee B., Kim B. M., Raouf R., Shin Y. K., Oh U. (2008) TMEM16A confers receptor-activated calcium-dependent chloride conductance. Nature 455, 1210–1215 - PubMed
-
- Caputo A., Caci E., Ferrera L., Pedemonte N., Barsanti C., Sondo E., Pfeffer U., Ravazzolo R., Zegarra-Moran O., Galietta L. J. (2008) TMEM16A, a membrane protein associated with calcium-dependent chloride channel activity. Science 322, 590–594 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HL73856/HL/NHLBI NIH HHS/United States
- R01 EY013574/EY/NEI NIH HHS/United States
- DK86125/DK/NIDDK NIH HHS/United States
- R01 EB000415/EB/NIBIB NIH HHS/United States
- R01 DK035124/DK/NIDDK NIH HHS/United States
- DK72517/DK/NIDDK NIH HHS/United States
- EY13574/EY/NEI NIH HHS/United States
- DK35124/DK/NIDDK NIH HHS/United States
- R01 HL073856/HL/NHLBI NIH HHS/United States
- P30 DK072517/DK/NIDDK NIH HHS/United States
- RC1 DK086125/DK/NIDDK NIH HHS/United States
- EB00415/EB/NIBIB NIH HHS/United States
- R37 DK035124/DK/NIDDK NIH HHS/United States
- R37 EB000415/EB/NIBIB NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

