Activation of the annexin A1 pathway underlies the protective effects exerted by estrogen in polymorphonuclear leukocytes
- PMID: 21836070
- PMCID: PMC3357483
- DOI: 10.1161/ATVBAHA.111.235176
Activation of the annexin A1 pathway underlies the protective effects exerted by estrogen in polymorphonuclear leukocytes
Abstract
Objective: The anti-inflammatory properties of the female sex hormone estrogen have been linked to a reduced incidence of cardiovascular disease. In the present study, we addressed whether estrogen could activate vasculoprotective mechanisms via annexin A1 (AnxA1) mobilization in human polymorphonuclear cells (PMNs).
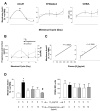
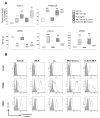
Methods and results: Using whole-blood flow cytometry, we demonstrated that premenopausal women expressed higher levels of surface AnxA1 on circulating PMNs compared with males. This correlated with high plasma estrogen during the menstrual cycle. The addition of estrogen in vitro to male PMNs induced rapid mobilization of AnxA1, optimal at 5 ng/mL and a 30-minute incubation period; this effect was abolished in the presence of the estrogen receptor antagonist ICI182780. Estrogen addition to human PMNs induced a distinct AnxA1(hi) CD62L(lo) CD11b(lo) phenotype, and this was associated with lower cell activation as measured by microparticle formation. Treatment of human PMNs with E(2) inhibited cell adhesion to an endothelial cell monolayer under shear, which was absent when endogenous AnxA1 was neutralized. Of interest, addition of estrogen to PMNs flowed over the endothelial monolayer amplified its upregulation of AnxA1 localization on the cell surface. Finally, in a model of intravital microscopy, estrogen inhibition of white blood cell adhesion to the postcapillary venule was absent in mice nullified for AnxA1.
Conclusion: We unveil a novel AnxA1-dependent mechanism behind the inhibitory properties of estrogen on PMN activation, describing a novel phenotype with a conceivable impact on the vasculoprotective effects of this hormone.
Figures




References
-
- Kannel WB, Wilson P. Risk factors that attenuate the female coronary disease advantage. Arch Intern Med. 1995;155:57–61. - PubMed
-
- Bakir S, Mori T, Durand J, Chen YF, Oparil S. Estrogen-induced vasoprotection is estrogen receptor dependent: evidence from the balloon-injured rat carotid artery mode. Circulation. 2000;101:2342–2344. - PubMed
-
- Chen SJ, Li H, Durand J, Oparil S, Chen Y. Estrogen reduces myointimal proliferation after balloon injury of rat carotid artery. Circulation. 1996;93:577–584. - PubMed
-
- Sherwood A, Bower JK, McFetridge-Durdle J, Blumenthal JA, Newby LK, Hinderliter A. Age moderates the short-term effects of transdermal 17beta-estradiol on endothelium-dependent vascular function in postmenopausal women. Arterioscler Thromb Vasc. 2007;27:1782–1787. - PubMed
-
- Hayashi T, Yamada K, Esaki T, Kuzuya M, Satake S, Ishikawa T, Hidaka H, Iguchi A. Estrogen increases endothelial nitric oxide by a receptor-mediated system. Biochem Biophys. 1995;214:847–855. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

