Primary bimorphic adrenocortical disease: cause of hypercortisolism in McCune-Albright syndrome
- PMID: 21836496
- PMCID: PMC4140081
- DOI: 10.1097/PAS.0b013e31821ec4ce
Primary bimorphic adrenocortical disease: cause of hypercortisolism in McCune-Albright syndrome
Abstract
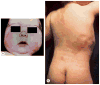
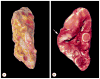
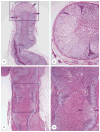
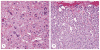
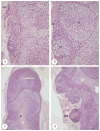
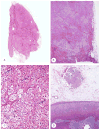
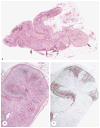
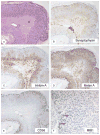
McCune-Albright syndrome (polyostotic fibrous dysplasia, café-au-lait skin spots, and precocious puberty) is a genetically mosaic disorder with populations of mutant and normal cells in affected organs. Cushing syndrome, a rare feature of the condition, usually affects infants and is the result of corticotropin-independent primary bilateral adrenal disease, usually interpreted as nodular adrenocortical hyperplasia. In this study of 9 patients with Cushing syndrome and McCune-Albright syndrome, light microscopy revealed a characteristic bimorphic pattern of diffuse and nodular hyperplasia and a distinctive form of cortical atrophy with apparent zona glomerulosa hyperplasia in 8 patients, all very young. The pattern could be explained by the presence of a mosaic distribution of mutant and normal cells in the adrenal glands. The findings are different from those in inherited or other forms of genetically caused Cushing syndrome. The ninth patient, aged 17 years, had an adrenal adenoma and diffuse cortical hyperplasia in each adrenal gland.
Conflict of interest statement
Conflicts of Interest and Sources of Funding: J.A.C. received funding from the Mayo Foundation and the Intramural Program, NIH project Z01HD00064204.
Figures


References
-
- Aarskog D, Tveteraas E. McCune-Albright’s syndrome following adrenalectomy for Cushing syndrome in infancy. J Pediatr. 1968;73:89–96. - PubMed
-
- Albright F, Butler AM, Bloomberg E. Rickets resistant to vitamin D therapy. Am J Dis Child. 1937;54:529–547.
-
- Albright F, Butler AM, Hampton AO, et al. Syndrome characterized by osteitis fibrosa disseminata, areas of pigmentation and endocrine dysfunction, with precocious puberty in females: report of five cases. N Engl J Med. 1937;216:727–746.
-
- Beckwith JB. Macroglossia, omphalocele, adrenal cytomegaly, gigantism, and hyperplastic visceromegaly. Birth Defects. 1969;5:188–196.
-
- Benjamin DR, McRoberts JW. Polyostotic fibrous dysplasia associated with Cushing syndrome. Arch Pathol. 1973;96:175–178. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

