The executioners sing a new song: killer caspases activate microglia
- PMID: 21836616
- PMCID: PMC3190105
- DOI: 10.1038/cdd.2011.107
The executioners sing a new song: killer caspases activate microglia
Abstract
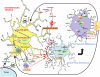
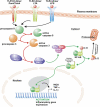
Activation of microglia and inflammation-mediated neurotoxicity are suggested to have key roles in the pathogenesis of several neurodegenerative disorders. We recently published an article in Nature revealing an unexpected role for executioner caspases in the microglia activation process. We showed that caspases 8 and 3/7, commonly known to have executioner roles for apoptosis, can promote microglia activation in the absence of death. We found these caspases to be activated in microglia of PD and AD subjects. Inhibition of this signaling pathway hindered microglia activation and importantly reduced neurotoxicity in cell and animal models of disease. Here we review evidence suggesting that microglia can have a key role in the pathology of neurodegenerative disorders. We discuss possible underlying mechanisms regulating their activation and neurotoxic effect. We focus on the provocative hypothesis that caspase inhibition can be neuroprotective by targeting the microglia rather than the neurons themselves.
Figures

Similar articles
-
Caspase signalling controls microglia activation and neurotoxicity.Nature. 2011 Apr 21;472(7343):319-24. doi: 10.1038/nature09788. Epub 2011 Mar 9. Nature. 2011. PMID: 21389984
-
Regulation of caspase-3 processing by cIAP2 controls the switch between pro-inflammatory activation and cell death in microglia.Cell Death Dis. 2014 Dec 11;5(12):e1565. doi: 10.1038/cddis.2014.514. Cell Death Dis. 2014. PMID: 25501826 Free PMC article.
-
New Views on the Misconstrued: Executioner Caspases and Their Diverse Non-apoptotic Roles.Neuron. 2015 Nov 4;88(3):461-74. doi: 10.1016/j.neuron.2015.08.029. Neuron. 2015. PMID: 26539888 Review.
-
Involvement of proinflammatory factors, apoptosis, caspase-3 activation and Ca2+ disturbance in microglia activation-mediated dopaminergic cell degeneration.Mech Ageing Dev. 2005 Dec;126(12):1241-54. doi: 10.1016/j.mad.2005.06.012. Epub 2005 Aug 19. Mech Ageing Dev. 2005. PMID: 16112714
-
Caspases orchestrate microglia instrumental functions.Prog Neurobiol. 2018 Dec;171:50-71. doi: 10.1016/j.pneurobio.2018.09.007. Epub 2018 Oct 2. Prog Neurobiol. 2018. PMID: 30290215 Review.
Cited by
-
Chronic stress enhances microglia activation and exacerbates death of nigral dopaminergic neurons under conditions of inflammation.J Neuroinflammation. 2014 Feb 24;11:34. doi: 10.1186/1742-2094-11-34. J Neuroinflammation. 2014. PMID: 24565378 Free PMC article.
-
Anti-proliferative effects of tricyclodecan-9-yl-xanthogenate (D609) involve ceramide and cell cycle inhibition.Mol Neurobiol. 2012 Jun;45(3):455-64. doi: 10.1007/s12035-012-8254-0. Epub 2012 Mar 14. Mol Neurobiol. 2012. PMID: 22415444 Free PMC article.
-
Adenosine A1 Receptor Agonist (R-PIA) before Pilocarpine Modulates Pro- and Anti-Apoptotic Factors in an Animal Model of Epilepsy.Pharmaceuticals (Basel). 2021 Apr 18;14(4):376. doi: 10.3390/ph14040376. Pharmaceuticals (Basel). 2021. PMID: 33919533 Free PMC article.
-
Betaine Inhibits Interleukin-1β Production and Release: Potential Mechanisms.Front Immunol. 2018 Nov 20;9:2670. doi: 10.3389/fimmu.2018.02670. eCollection 2018. Front Immunol. 2018. PMID: 30515160 Free PMC article. Review.
-
The Role of Caspase Family in Acute Brain Injury: The Potential Therapeutic Targets in the Future.Curr Neuropharmacol. 2022;20(6):1194-1211. doi: 10.2174/1570159X19666211111121146. Curr Neuropharmacol. 2022. PMID: 34766893 Free PMC article. Review.
References
-
- Rio-Hortega Pd. El ‘tercer elemento' de los centros nerviosos. Poder fagocitario y movilidad de la microglia. Bol de la Soc espanola de Biol. 1919;Ano ix:154–166.
-
- Obeso JA, Rodriguez-Oroz MC, Rodriguez M, Lanciego JL, Artieda J, Gonzalo N, et al. Pathophysiology of the basal ganglia in Parkinson's disease. Trends Neurosci. 2000;23 (10 Suppl:S8–19. - PubMed
-
- Mouradian MM. Recent advances in the genetics and pathogenesis of Parkinson disease. Neurology. 2002;58:179–185. - PubMed
-
- Langston JW, Forno LS, Tetrud J, Reeves AG, Kaplan JA, Karluk D. Evidence of active nerve cell degeneration in the substantia nigra of humans years after 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine exposure. Ann Neurol. 1999;46:598–605. - PubMed
-
- McGeer PL, Itagaki S, McGeer EG. Expression of the histocompatibility glycoprotein HLA-DR in neurological disease. Acta Neuropathol. 1988;76:550–557. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

