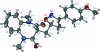3-{[3-(4-Meth-oxy-phen-yl)-4,5-dihydro-1,2-oxazol-5-yl]meth-yl}-1,5-dimethyl-1H-1,5-benzodiazepine-2,4(3H,5H)-dione
- PMID: 21837118
- PMCID: PMC3151967
- DOI: 10.1107/S1600536811022872
3-{[3-(4-Meth-oxy-phen-yl)-4,5-dihydro-1,2-oxazol-5-yl]meth-yl}-1,5-dimethyl-1H-1,5-benzodiazepine-2,4(3H,5H)-dione
Abstract
The mol-ecule of the title compound, C(22)H(23)N(3)O(4), features a benzodiazepine fused-ring system whose seven-membered ring adopts a boat-shaped conformation (with the C atoms of the fused-ring as the stern and the methine C atom as the prow). The methyl-ene C atom connected to the methine C atom occupies an equatorial position. The methyl-ene C atom is connected to the five-membered oxazole ring, both of which are disordered over two positions in a 0.634 (4):0.366 (4) ratio. Weak inter-molecular C-H⋯O hydrogen bonding is present in the crystal structure.
Figures
Similar articles
-
3-{[3-(4-Chloro-phen-yl)-4,5-dihydro-1,2-oxazol-5-yl]meth-yl}-1,5-dimethyl-1H-1,5-benzodiazepine-2,4(3H,5H)-dione.Acta Crystallogr Sect E Struct Rep Online. 2011 Feb 26;67(Pt 3):o720. doi: 10.1107/S160053681100657X. Acta Crystallogr Sect E Struct Rep Online. 2011. PMID: 21522462 Free PMC article.
-
3-(12-Bromo-dodec-yl)-1,5-dimethyl-1H-1,5-benzodiazepine-2,4(3H,5H)-dione.Acta Crystallogr Sect E Struct Rep Online. 2010 Oct 13;66(Pt 11):o2805. doi: 10.1107/S1600536810040134. Acta Crystallogr Sect E Struct Rep Online. 2010. PMID: 21588999 Free PMC article.
-
3-(6-Bromo-hex-yl)-1,5-dimethyl-1H-1,5-benzodiazepine-2,4(3H,5H)-dione.Acta Crystallogr Sect E Struct Rep Online. 2010 Oct 13;66(Pt 11):o2804. doi: 10.1107/S1600536810040122. Acta Crystallogr Sect E Struct Rep Online. 2010. PMID: 21588998 Free PMC article.
-
1,5-Dimethyl-3-[(3-phenyl-4,5-dihydro-1,2-oxazol-5-yl)meth-yl]-1H-1,5-benzodiazepine-2,4(3H,5H)-dione.Acta Crystallogr Sect E Struct Rep Online. 2010 Oct 30;66(Pt 11):o2983. doi: 10.1107/S1600536810042972. Acta Crystallogr Sect E Struct Rep Online. 2010. PMID: 21589149 Free PMC article.
-
Recyclization of 5-Amino- oxazoles as a Route to new Functionalized Heterocycles (Developments of V.P. Kukhar Institute of Bioorganic Chemistry and Petrochemistry of the NAS of Ukraine).Chem Rec. 2024 Feb;24(2):e202300264. doi: 10.1002/tcr.202300264. Epub 2023 Oct 26. Chem Rec. 2024. PMID: 37882374 Review.
References
-
- Barbour, L. J. (2001). J. Supramol. Chem. 1, 189–191.
-
- Bruker (2005). APEX2 and SAINT Bruker AXS Inc., Madison, Wisconsin, USA.
-
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. - PubMed
-
- Westrip, S. P. (2010). J. Appl. Cryst. 43, 920–925.
LinkOut - more resources
Full Text Sources