A high-performance microsystem for isolating circulating tumor cells
- PMID: 21837324
- PMCID: PMC6765387
- DOI: 10.1039/c1lc20331b
A high-performance microsystem for isolating circulating tumor cells
Abstract
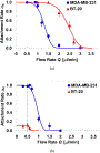
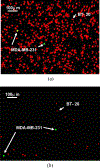
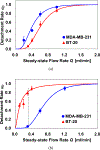
A unique flow field pattern in a bio-functional microchannel is utilized to significantly enhance the performance of a microsystem developed for selectively isolating circulating tumor cells from cell suspensions. For high performance of such systems, disposal of maximum non-target species is just as important as retention of maximum target species; unfortunately, most studies ignore or fail to report this aspect. Therefore, sensitivity and specificity are introduced as quantitative criteria to evaluate the system performance enabling a direct comparison among systems employing different techniques. The newly proposed fluidic scheme combines a slow flow field, for maximum target-cell attachment, followed by a faster flow field, for maximum detachment of non-target cells. Suspensions of homogeneous or binary mixtures of circulating breast tumor cells, with varying relative concentrations, were driven through antibody-functionalized microchannels. Either EpCAM or cadherin-11 transmembrane receptors were targeted to selectively capture target cells from the suspensions. Cadherin-11-expressing MDA-MB-231 cancer cells were used as target cells, while BT-20 cells were used as non-target cells as they do not express cadherin-11. The attachment and detachment of these two cell lines are characterized, and a two-step attachment/detachment flow field pattern is implemented to enhance the system performance in capturing target cells from binary mixtures. While the system sensitivity remains high, above 0.95, the specificity increases from about 0.85 to 0.95 solely due to the second detachment step even for a 1 : 1000 relative concentration of the target cells.
Figures



References
-
- Cristofanilli M, Budd GT, Ellis MJ, Stopeck A, Matera J, Miller MC, Reuben JM, Doyle GV, Allard WJ, Terstappen LW, and Hayes DF, Circulating tumor cells, disease progression, and survival in metastatic breast cancer. New Engl. J. Med, 2004, 351, 781–791. - PubMed
-
- Cristofanilli M, Broglio KR, Guarneri V, Jackson S, Fritsche HA, Islam R, Dawood S, Reuben JM, Kau SW, Lara JM, Krishnamurthy S, Ueno NT, Hortobagyi GN, and Valero V, Circulating tumor cells in metastatic breast cancer: biologic staging beyond tumor burden. Clin. Breast Cancer, 2007, 7, 471–479. - PubMed
-
- Kahn HJ, Presta A, Yang L-Y, Blondal J, Trudeau M, Lickley L, Holloway C, McCready DR, Maclean D and Marks A, Enumeration of circulating tumor cells in the blood of breast cancer patients after filtration enrichment: correlation with disease stage. Breast Cancer Res. Tr., 2004, 86, 237–247. - PubMed
-
- Chang WC, Lee LP and Liepmann D, Biomimetic technique for adhesion-based collection and separation of cells in a microfluidic channel. Lab Chip, 2005, 5, 64–73. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

