Use of human embryonic stem cell derived-mesenchymal cells for cardiac repair
- PMID: 21837664
- PMCID: PMC3220775
- DOI: 10.1002/bit.23301
Use of human embryonic stem cell derived-mesenchymal cells for cardiac repair
Abstract
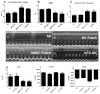
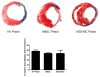
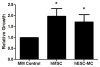
Human mesenchymal stem cells (hMSC) have proven beneficial in the repair and preservation of infarcted myocardium. Unfortunately, MSCs represent a small portion of the bone marrow and require ex vivo expansion. To further advance the clinical usefulness of cellular cardiomyoplasty, derivation of "MSC-like" cells that can be made available "off-the-shelf" are desirable. Recently, human embryonic stem cell-derived mesenchymal cells (hESC-MC) were described. We investigated the efficacy of hESC-MC for cardiac repair after myocardial infarction (MI) compared to hMSC. Because of increased efficacy of cell delivery, cells were embedded into collagen patches and delivered to infarcted myocardium. Culture of hMSC and hESC-MCs in collagen patches did not induce differentiation or significant loss in viability. Transplantation of hMSC and hES-MC patches onto infarcted myocardium of athymic nude rats prevented adverse changes in infarct wall thickness and fractional area change compared to a non-viable patch control. Hemodynamic assessment showed that hMSCs and hES-MC patch application improved end diastolic pressure equivalently. There were no changes in systolic function. hES-MC and hMSC construct application enhanced neovessel formation compared to a non-viable control, and each cell type had similar efficacy in stimulating endothelial cell growth in vitro. In summary, the use of hES-MC provides similar efficacy for cellular cardiomyoplasty as compared to hMSC and may be considered a suitable alternative for cell therapy.
Copyright © 2011 Wiley Periodicals, Inc.
Figures



Similar articles
-
Modulation of human mesenchymal stem cell function in a three-dimensional matrix promotes attenuation of adverse remodelling after myocardial infarction.J Tissue Eng Regen Med. 2013 Mar;7(3):192-202. doi: 10.1002/term.511. Epub 2011 Nov 18. J Tissue Eng Regen Med. 2013. PMID: 22095744
-
Chitosan/silk fibroin modified nanofibrous patches with mesenchymal stem cells prevent heart remodeling post-myocardial infarction in rats.Acta Biomater. 2018 Oct 15;80:154-168. doi: 10.1016/j.actbio.2018.09.013. Epub 2018 Sep 13. Acta Biomater. 2018. PMID: 30218777
-
A tissue engineering approach to progenitor cell delivery results in significant cell engraftment and improved myocardial remodeling.Stem Cells. 2007 Sep;25(9):2350-7. doi: 10.1634/stemcells.2007-0132. Epub 2007 May 24. Stem Cells. 2007. PMID: 17525236 Free PMC article.
-
Mesenchymal stem cells and their potential as cardiac therapeutics.Circ Res. 2004 Jul 9;95(1):9-20. doi: 10.1161/01.RES.0000135902.99383.6f. Circ Res. 2004. PMID: 15242981 Review.
-
Cellular cardiomyoplasty and cardiac tissue engineering for myocardial therapy.Adv Drug Deliv Rev. 2010 Jun 15;62(7-8):784-97. doi: 10.1016/j.addr.2010.03.001. Epub 2010 Mar 6. Adv Drug Deliv Rev. 2010. PMID: 20214939 Review.
Cited by
-
A continuum model and simulations for large deformation of anisotropic fiber-matrix composites for cardiac tissue engineering.J Mech Behav Biomed Mater. 2021 Sep;121:104627. doi: 10.1016/j.jmbbm.2021.104627. Epub 2021 Jun 7. J Mech Behav Biomed Mater. 2021. PMID: 34130078 Free PMC article.
-
iPSC-derived human mesenchymal stem cells improve myocardial strain of infarcted myocardium.J Cell Mol Med. 2014 Aug;18(8):1644-54. doi: 10.1111/jcmm.12351. Epub 2014 Jun 28. J Cell Mol Med. 2014. PMID: 24974908 Free PMC article.
-
Mesenchymal stem cells in tumor development: emerging roles and concepts.Cell Adh Migr. 2012 May-Jun;6(3):220-30. doi: 10.4161/cam.20875. Epub 2012 May 1. Cell Adh Migr. 2012. PMID: 22863739 Free PMC article. Review.
-
Improved Left Ventricular Aneurysm Repair with Cell- and Cytokine-Seeded Collagen Patches.Stem Cells Int. 2018 Feb 13;2018:4717802. doi: 10.1155/2018/4717802. eCollection 2018. Stem Cells Int. 2018. PMID: 29531539 Free PMC article.
-
A novel platelet lysate hydrogel for endothelial cell and mesenchymal stem cell-directed neovascularization.Acta Biomater. 2016 May;36:86-98. doi: 10.1016/j.actbio.2016.03.002. Epub 2016 Mar 4. Acta Biomater. 2016. PMID: 26961805 Free PMC article.
References
-
- Assmus B, Honold J, Schachinger V, Britten MB, Fischer-Rasokat U, Lehmann R, Teupe C, Pistorius K, Martin H, Abolmaali ND, et al. Transcoronary transplantation of progenitor cells after myocardial infarction. N Engl J Med. 2006;355(12):1222–32. - PubMed
-
- Berry MF, Engler AJ, Woo YJ, Pirolli TJ, Bish LT, Jayasankar V, Morine KJ, Gardner TJ, Discher DE, Sweeney HL. Mesenchymal stem cell injection after myocardial infarction improves myocardial compliance. Am J Physiol Heart Circ Physiol. 2006;290(6):H2196–203. - PubMed
-
- Breland UM, Halvorsen B, Hol J, Oie E, Paulsson-Berne G, Yndestad A, Smith C, Otterdal K, Hedin U, Waehre T, et al. A potential role of the CXC chemokine GROalpha in atherosclerosis and plaque destabilization: downregulatory effects of statins. Arterioscler Thromb Vasc Biol. 2008;28(5):1005–11. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

