Mouse model of chronic post-arthroplasty infection: noninvasive in vivo bioluminescence imaging to monitor bacterial burden for long-term study
- PMID: 21837686
- PMCID: PMC3217109
- DOI: 10.1002/jor.21519
Mouse model of chronic post-arthroplasty infection: noninvasive in vivo bioluminescence imaging to monitor bacterial burden for long-term study
Abstract
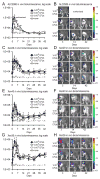
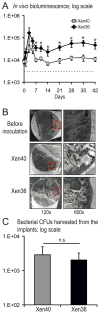
Post-arthroplasty infections are a devastating problem in orthopaedic surgery. While acute infections can be treated with a single stage washout and liner exchange, chronic infections lead to multiple reoperations, prolonged antibiotic courses, extended disability, and worse clinical outcomes. Unlike previous mouse models that studied an acute infection, this work aimed to develop a model of a chronic post-arthroplasty infection. To achieve this, a stainless steel implant in the knee joints of mice was inoculated with a bioluminescent Staphylococcus aureus strain (1 × 10(2) -1 × 10(4) colony forming units, CFUs) and in vivo imaging was used to monitor the bacterial burden for 42 days. Four different S. aureus strains were compared in which the bioluminescent construct was integrated in an antibiotic selection plasmid (ALC2906), the bacterial chromosome (Xen29 and Xen40), or a stable plasmid (Xen36). ALC2906 had increased bioluminescent signals through day 10, after which the signals became undetectable. In contrast, Xen29, Xen40, and Xen36 had increased bioluminescent signals through 42 days with the highest signals observed with Xen36. ALC2906, Xen29, and Xen40 induced significantly more inflammation than Xen36 as measured by in vivo enhanced green fluorescence protein (EGFP)-neutrophil flourescence of LysEGFP mice. All four strains induced comparable biofilm formation as determined by variable-pressure scanning electron microscopy. Using a titanium implant, Xen36 had higher in vivo bioluminescence signals than Xen40 but had similar biofilm formation and adherent bacteria. In conclusion, Xen29, Xen40, and especially Xen36, which had stable bioluminescent constructs, are feasible for long-term in vivo monitoring of bacterial burden and biofilm formation to study chronic post-arthroplasty infections and potential antimicrobial interventions.
Copyright © 2011 Orthopaedic Research Society.
Figures


References
-
- Kurtz SM, Lau E, Schmier J, et al. Infection burden for hip and knee arthroplasty in the United States. J Arthroplasty. 2008;23:984–991. - PubMed
-
- Bozic KJ, Kurtz SM, Lau E, et al. The epidemiology of revision total hip arthroplasty in the United States. J Bone Joint Surg Am. 2009;91:128–133. - PubMed
-
- Kurtz S, Ong K, Lau E, et al. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89:780–785. - PubMed
-
- Kurtz SM, Ong KL, Schmier J, et al. Future clinical and economic impact of revision total hip and knee arthroplasty. J Bone Joint Surg Am. 2007;89(Suppl 3):144–151. - PubMed
-
- Fulkerson E, Valle CJ, Wise B, et al. Antibiotic susceptibility of bacteria infecting total joint arthroplasty sites. J Bone Joint Surg Am. 2006;88:1231–1237. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

