Activity-based protein profiling of protein arginine methyltransferase 1
- PMID: 21838253
- PMCID: PMC3199286
- DOI: 10.1021/cb2001473
Activity-based protein profiling of protein arginine methyltransferase 1
Abstract
The protein arginine methyltransferases (PRMTs) are SAM-dependent enzymes that catalyze the mono- and dimethylation of peptidyl arginine residues. PRMT1 is the founding member of the PRMT family, and this isozyme is responsible for methylating ∼85% of the arginine residues in mammalian cells. Additionally, PRMT1 activity is aberrantly upregulated in heart disease and cancer. As a part of a program to develop isozyme-specific PRMT inhibitors, we recently described the design and synthesis of C21, a chloroacetamidine bearing histone H4 tail analogue that acts as an irreversible PRMT1 inhibitor. Given the covalent nature of the interaction, we set out to develop activity-based probes (ABPs) that could be used to characterize the physiological roles of PRMT1. Herein, we report the design, synthesis, and characterization of fluorescein-conjugated C21 (F-C21) and biotin-conjugated C21 (B-C21) as PRMT1-specific ABPs. Additionally, we provide the first evidence that PRMT1 activity is negatively regulated in a spatial and temporal fashion.
Figures
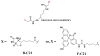



References
-
- Lin WJ, Gary JD, Yang MC, Clarke S, Herschman HR. The mammalian immediate-early TIS21 protein and the leukemia-associated BTG1 protein interact with a protein-arginine N-methyltransferase. J Biol Chem. 1996;271:15034–15044. - PubMed
-
- McBride AE, Silver PA. State of the arg: protein methylation at arginine comes of age. Cell. 2001;106:5–8. - PubMed
-
- Friesen WJ, Massenet S, Paushkin S, Wyce A, Dreyfuss G. SMN, the product of the spinal muscular atrophy gene, binds preferentially to dimethylarginine-containing protein targets. Mol Cell. 2001;7:1111–1117. - PubMed
-
- Tran CT, Leiper JM, Vallance P. The DDAH/ADMA/NOS pathway. Atheroscler. 2003;(Suppl 4):33–40. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

