From mechanism to mouse: a tale of two bioorthogonal reactions
- PMID: 21838330
- PMCID: PMC3184615
- DOI: 10.1021/ar200148z
From mechanism to mouse: a tale of two bioorthogonal reactions
Abstract
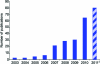
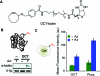
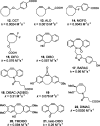
Bioorthogonal reactions are chemical reactions that neither interact with nor interfere with a biological system. The participating functional groups must be inert to biological moieties, must selectively reactive with each other under biocompatible conditions, and, for in vivo applications, must be nontoxic to cells and organisms. Additionally, it is helpful if one reactive group is small and therefore minimally perturbing of a biomolecule into which it has been introduced either chemically or biosynthetically. Examples from the past decade suggest that a promising strategy for bioorthogonal reaction development begins with an analysis of functional group and reactivity space outside those defined by Nature. Issues such as stability of reactants and products (particularly in water), kinetics, and unwanted side reactivity with biofunctionalities must be addressed, ideally guided by detailed mechanistic studies. Finally, the reaction must be tested in a variety of environments, escalating from aqueous media to biomolecule solutions to cultured cells and, for the most optimized transformations, to live organisms. Work in our laboratory led to the development of two bioorthogonal transformations that exploit the azide as a small, abiotic, and bioinert reaction partner: the Staudinger ligation and strain-promoted azide-alkyne cycloaddition. The Staudinger ligation is based on the classic Staudinger reduction of azides with triarylphosphines first reported in 1919. In the ligation reaction, the intermediate aza-ylide undergoes intramolecular reaction with an ester, forming an amide bond faster than aza-ylide hydrolysis would otherwise occur in water. The Staudinger ligation is highly selective and reliably forms its product in environs as demanding as live mice. However, the Staudinger ligation has some liabilities, such as the propensity of phosphine reagents to undergo air oxidation and the relatively slow kinetics of the reaction. The Staudinger ligation takes advantage of the electrophilicity of the azide; however, the azide can also participate in cycloaddition reactions. In 1961, Wittig and Krebs noted that the strained, cyclic alkyne cyclooctyne reacts violently when combined neat with phenyl azide, forming a triazole product by 1,3-dipolar cycloaddition. This observation stood in stark contrast to the slow kinetics associated with 1,3-dipolar cycloaddition of azides with unstrained, linear alkynes, the conventional Huisgen process. Notably, the reaction of azides with terminal alkynes can be accelerated dramatically by copper catalysis (this highly popular Cu-catalyzed azide-alkyne cycloaddition (CuAAC) is a quintessential "click" reaction). However, the copper catalysts are too cytotoxic for long-term exposure with live cells or organisms. Thus, for applications of bioorthogonal chemistry in living systems, we built upon Wittig and Krebs' observation with the design of cyclooctyne reagents that react rapidly and selectively with biomolecule-associated azides. This strain-promoted azide-alkyne cycloaddition is often referred to as "Cu-free click chemistry". Mechanistic and theoretical studies inspired the design of a series of cyclooctyne compounds bearing fluorine substituents, fused rings, and judiciously situated heteroatoms, with the goals of optimizing azide cycloaddition kinetics, stability, solubility, and pharmacokinetic properties. Cyclooctyne reagents have now been used for labeling azide-modified biomolecules on cultured cells and in live Caenorhabditis elegans, zebrafish, and mice. As this special issue testifies, the field of bioorthogonal chemistry is firmly established as a challenging frontier of reaction methodology and an important new instrument for biological discovery. The above reactions, as well as several newcomers with bioorthogonal attributes, have enabled the high-precision chemical modification of biomolecules in vitro, as well as real-time visualization of molecules and processes in cells and live organisms. The consequence is an impressive body of new knowledge and technology, amassed using a relatively small bioorthogonal reaction compendium. Expansion of this toolkit, an effort that is already well underway, is an important objective for chemists and biologists alike.
© 2011 American Chemical Society
Figures

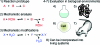





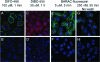
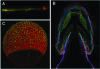
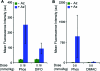
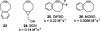
References
-
- Rideout D.; Calogeropoulou T.; Jaworski J.; McCarthy M. Synergism through Direct Covalent Bonding between Agents: A Strategy for Rational Design of Chemotherapeutic Combinations. Biopolymers 1990, 29, 247–262. - PubMed
-
- Griffin B. A.; Adams S. R.; Tsien R. Y. Specific Covalent Labeling of Recombinant Protein Molecules inside Live Cells. Science 1998, 281, 269–272. - PubMed
-
- Saxon E.; Bertozzi C. R. Cell Surface Engineering by a Modified Staudinger Reaction. Science 2000, 287, 2007–2010. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

