Treatment of advanced leukemia in mice with mRNA engineered T cells
- PMID: 21838572
- PMCID: PMC3237694
- DOI: 10.1089/hum.2011.070
Treatment of advanced leukemia in mice with mRNA engineered T cells
Abstract
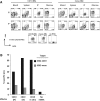
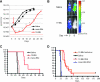
Cytotoxic T lymphocytes (CTLs) modified with chimeric antigen receptors (CARs) for adoptive immunotherapy of hematologic malignancies are effective in preclinical models and are being tested in several clinical trials. Although CTLs bearing stably expressed CARs generated by integrating viral vectors are efficacious and have potential long-term persistence, this mechanism of CAR expression can potentially result in significant toxicity. T cells were electroporated with an optimized in vitro transcribed RNA encoding a CAR against CD19. These RNA CAR CTLs were then tested in vitro and in vivo for efficacy. We found that T cells expressing an anti-CD19 CAR introduced by electroporation with optimized mRNA were potent and specific killers of CD19 target cells. CD19 RNA CAR T cells given to immunodeficient mice bearing xenografted leukemia rapidly migrated to sites of disease and retained significant target-specific lytic activity. Unexpectedly, a single injection of CD19 RNA CAR T cells reduced disease burden within 1 day after administration, resulting in a significant prolongation of survival in an aggressive leukemia xenograft model. The surface expression of the RNA CARs may be titrated, giving T cells with potentially tunable levels of effector functions such as cytokine release and cytotoxicity. RNA CARs are a genetic engineering approach that should not be subject to genotoxicity, and they provide a platform for rapidly optimizing CAR design before proceeding to more costly and laborious stable expression systems.
Figures



References
-
- Betts M.R. Koup R.A. Detection of T-cell degranulation: CD107a and b. Methods Cell Biol. 2004;75:497–512. - PubMed
-
- Birkholz K. Hombach A. Krug C., et al. Transfer of mRNA encoding recombinant immunoreceptors reprograms CD4+ and CD8+ T cells for use in the adoptive immunotherapy of cancer. Gene Ther. 2009;16:596–604. - PubMed
-
- Brentjens R. J. Santos E. Nikhamin Y., et al. Genetically targeted T cells eradicate systemic acute lymphoblastic leukemia xenografts. Clin. Cancer Res. 2007;13(18 Pt 1):5426–5435. - PubMed
-
- Brocker T. Karjalainen K. Adoptive tumor immunity mediated by lymphocytes bearing modified antigen-specific receptors. Adv. Immunol. 1998;68:257–269. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

